Published On November 20, 2020
After nearly a year living under a pandemic which has come to color every aspect of daily life, it’s easy to forget that the whole thing arose rather quietly. In early January, news broke of an outbreak of pneumonia in Wuhan, China, and began to trickle out to an international audience. On January 11, Chinese authorities announced that a 61-year-old was the first person to die in the outbreak, which had been raging for at least a month. On the same day, Chinese researchers released a draft genome sequence of the pathogen they believed was causing those illnesses—a new coronavirus. By January 20, health authorities had determined it could pass from person to person and, like other coronaviruses, probably had been transmitted to a human by an animal. That same day, the Centers for Disease Control and Prevention confirmed the first known U.S. case, a resident of Washington state.
In Boston, physicians and other health care workers at Massachusetts General Hospital listened closely to every dispatch. Responding to a pandemic is a scenario hospitals both dread and are built for. MGH, with its 209-year history, had encountered them more than once, although the staff had no living memory of the local outbreak of smallpox in 1851 or the 1918 global flu pandemic. The hospital did, however, regularly conduct drills to prepare for mass disasters and emergencies—not only contagious diseases but also active shooters, bioterrorism events, train wrecks, chemical spills and hurricanes. The hours of preparation had served them well whenever patients flooded in, as they did after the Boston Marathon bombing of 2013, a grisly event in the hospital’s history but one in which not a single admitted patient was lost.
Most hospitals drill for extreme scenarios in some way. But unlike most others, MGH had also been training for the previous five years to treat the world’s most dangerous infectious diseases. Following the 2014 outbreak of the Ebola virus in West Africa, the U.S. government designated MGH as one of 10 regional Ebola and other special pathogen treatment centers. This meant the hospital had to piece together protocols for the isolation, evaluation and management of patients with rare and deadly infectious diseases and also develop a special pathogens unit. The latter would include, among other tools, negative-pressure rooms to contain potentially airborne infectious agents, specialized personal protective equipment (PPE) and a dedicated team, says Erica Shenoy, medical director of the MGH regional treatment unit and associate chief of the hospital’s Infection Control Unit. In 2019, the hospital evaluated five suspected cases of Middle East respiratory syndrome (MERS). While this deadly coronovirus was ruled out in all five patients, no one suspected that it might be a dress rehearsal for the coming year.
Well before the pathogens unit would be needed, however, other teams were springing into action. When the genome of the new virus was made public, Boston-area researchers at Beth Israel Deaconess Medical Center, the Ragon Institute of MGH, MIT and Harvard, Massachusetts Eye and Ear and MGH began creating candidates for vaccines. Other researchers and scientists at MGH sorted through the literature to come up with possible treatments, while those who worked in community health asked questions about how a coronavirus pandemic might affect the Boston area and began laying the groundwork for a local response.

Source: Israel Vargas: Photos from MGH Photography and Getty Images
All of these efforts came as a community of 27,000 employees began to imagine worst-case outcomes and how they might bring their own expertise to bear. On January 27, the hospital made the call to activate its Hospital Incident Command System (HICS)—an organizational streamlining, mirroring a strategy used by public safety agencies in times of crisis. It brought coordination and a military-style precision to decisions that would ultimately upend every aspect of the hospital’s normal operations.
“At the start, we didn’t have enough information,” says Ann Prestipino, the HICS incident commander and an MGH senior vice president. Prestipino has spent her entire career at MGH, and previously was incident commander not only for the 2013 marathon bombing but also for the 2003 Station nightclub fire in West Warwick, R.I., which killed 100 people and injured more than 200, many of whom were rushed to MGH. This time, she says, “We were in active communication with colleagues around the world. We tried to learn as much as we could about how to keep our staff safe and to effectively take care of the wave of infected patients, who we knew were on the way.” Data and guidance did begin to flood in from federal and state health departments, but recommendations changed constantly, forcing HICS leaders to convene again and again—in the stately conference room that serves as the HICS command center during disasters—to revise their plans. “Key decisions had a shelf life of less than a week,” says Inga T. Lennes, senior vice president of Practice Improvement and Patient Experience at MGH.
During these early days, Katrina Armstrong, chair of the Department of Medicine and physician-in-chief at MGH, remembers sharing information in round-the-clock emails with her colleagues. One email stands out, a message from Bruce Walker, director of the Ragon Institute, who shared some of the frightening information coming from northern Italy. Walker was also talking with other Boston-area experts, including Harvard Medical School Dean George Daley, about how to jump-start collaboration across the local medical community, which included some of the most prominent research and biotech organizations in the world. He and an impressive cohort of health care leaders signed a letter to The Boston Globe on March 5, 2020, that stated: “No single institution is going to solve this problem.”
They had just called a meeting at Harvard Medical School to launch what would soon be named the Massachusetts Consortium on Pathogen Readiness—MassCPR—made up of representatives from leading universities, academic hospitals, biotechnology and pharmaceutical firms, research institutes, foundations and the Massachusetts Department of Public Health. This decision to work together formally was perhaps the most telling sign that the new virus would leave a lasting mark on the medical community. Consortium members, including medical titans that were normally rivals for patents and discoveries, agreed to share their data and research on diagnostic tools, treatments and vaccines. That kind of precedent-shattering cooperation would be essential in fighting the new disease that the World Health Organization had named COVID-19.
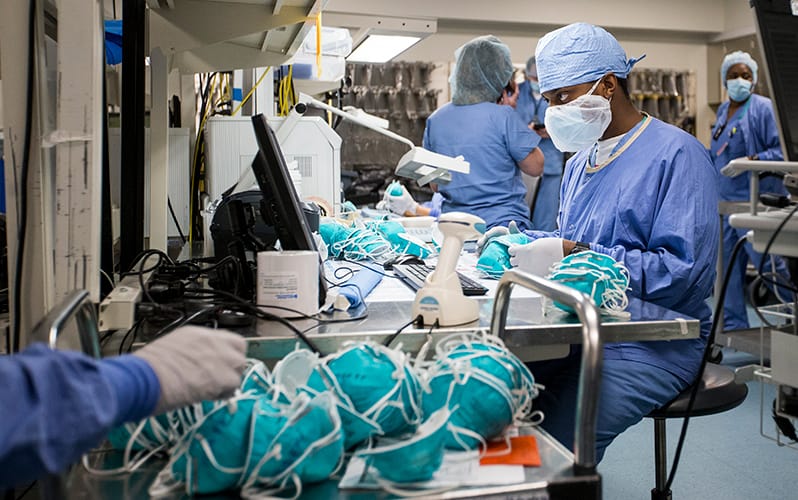
The team at MGH found a novel way to decontaminate the N95 respirators that were in short supply. This allowed thousands more to be in circulation. Source: Boston Globe/Getty Images
By the time of that March meeting, the virus was already roaring across Europe and overwhelming many hospitals, which found themselves desperately short of beds, ventilators and workers. At MGH, Peter Dunn, vice president of Perioperative Services and Healthcare Systems Engineering (HSE), was tasked with helping the hospital avoid the same fate. The first order of business was to estimate how many patients with COVID-19 might arrive at MGH, and when. At the pandemic’s peak, what resources would be needed for general care and how many people would land in the ICU? How many ventilators would be enough?
The HSE team includes not only doctors but also mathematicians, who use advanced systems modeling to calculate better health care delivery. In less harried times, their focus had been to create more efficient flows of patients into and out of the hospital, as when the team applied machine learning techniques to assign scores to patients after surgery, helping predict when they would be ready to go home. This model, published in JAMA Network last year, could speed up discharges and free beds for new patients.
The forecasting tools they needed now, however, would have to apply to a wholly new contagion about which very little was known. They found one source of data in Italian hospitals, which had been devastated by COVID-19 in February and early March. “This provided enough data to give us a head start on planning new surge units,” says Kyan Safavi, medical director of HSE and a critical care physician.
The bigger challenge was to determine when MGH and other hospitals in its network might hit their peak volume of COVID-19 patients—a number that would give them the upper target for beds and other resources. Safavi, Dunn and the rest of the HSE team adapted an epidemiologic model and applied different estimates for the virus’s behavior and infection rate as well as the proportion of infected people who might need to be hospitalized. They also calculated how many COVID-19 patients might need treatment in the ICU and how long they would need to stay, all based on ever-changing information that became available to them.
In their worst-case scenario, the Mass General Brigham hospital system—of which MGH is a part—looked like it could need 1,000 ICU beds for COVID-19 patients and an additional 1,500 general-care beds. “These estimates set a planning exercise in motion to ensure that all hospitals were prepared for the capacity, providers, nurses and other resources they might need,” Safavi says.
The incident command team saw that much of the hospital would have to be transformed on the fly. “Before COVID, I would have said you were out of your mind for even imagining that we could create 100 ICU beds in three weeks,” says Kathryn Hibbert, director of the Medical Intensive Care Unit. Yet, through what Hibbert describes as an “incredible, herculean team effort by infection control, nursing, respiratory therapy, materials management and physicians working around the clock,” MGH nearly doubled the hospital’s existing 133 intensive care beds. Pediatric, burn and neurosurgery ICUs were commandeered for the COVID-19 effort, with some of the patients who would ordinarily be cared for in those units diverted to other hospitals. New ICUs were created in post-operative recovery rooms that were no longer needed for patients after elective surgeries, which had been canceled.
To staff these new units, the hospital recruited any physician with critical care experience. But one of the most pressing needs was for nurses. Associate chief nurse Theresa Gallivan estimated that an additional 600 full-time critical care nurses would be required to meet the increased demand for ICU beds. To get there, a partnership model was employed and supported by nurses throughout the hospital to spread current critical care nurses across all of the ICU units and reassign general care nurses to work alongside them. The hospital also brought in as many travel nurses as it could. “This crisis partnership model, while challenging for all involved, enabled us to care for the high number of critically ill patients,” Gallivan says.
The new ICUs would be called into service on a just-in-time basis. “We didn’t want to waste resources by opening them too soon,” Dunn says. As COVID-19 cases rose, patients were admitted to the first additional ICU on March 14, with the other new facilities coming online as the patient count rose.
With a pandemic looming, the problem of reducing infection risk for hospital workers was on everyone’s mind—and addressing the coming shortages of personal protective equipment became a national riddle without good answers. “We were hearing about how deadly the virus was for health care workers, but we really didn’t know the best approach for protecting them,” Armstrong says.
It quickly became clear that one recommended measure—N95 respirators, face masks that filter out at least 95% of airborne particles—would be crucial. In mid-February, the hospital calculated that its cache of N95 masks and other PPE, meant to last two weeks, might be depleted even more quickly than that. New shipments from China, the main supplier of PPE, had slowed to a trickle, and 3M had stopped consistently shipping the hospital’s regular order of masks.
N95 respirators have to seal precisely, and clinicians undergo an elaborate fit test each year. By February’s end, however, the N95 inventory at MGH was depleted, and small sizes were becoming an acute supply issue. The hospital was able, with the help of other hospitals within Mass General Brigham, to procure additional N95s, but the newer models would require another 5,000 fit tests. More N95s would be needed as patient numbers went up.
Finding more N95s fell to Ed Raeke, director of Materials Management at MGH, whose job is to see that supplies arrive at the right time and place. As shortages mounted, Raeke fielded hundreds of offers of N95s—mostly by email—from people who claimed to have a connection in China or to know someone who had access to the masks. When a legitimate offer could be locked down, the hospital might have to pay $4 to $8 a mask, compared with less than $1 pre-pandemic. Without a steady and reliable new source, the hospital would need to find a way to reuse the masks it had. The question was how to do this safely.
Scientists in the lab of Orhun Muratoglu, director of the Harris Orthopaedics Laboratory and the Technology Implementation Research Center at MGH, pivoted their research from developing hip joint implants to decontaminating used N95 respirators. That wasn’t a huge leap, curiously, because it was related to a problem that Muratoglu encountered regularly. “There are many ways to sterilize an implant, and we threw all of them at the N95 masks,” he says.
The first few approaches—discussed on daily Zoom calls with more than 100 participants around the world—degraded the fit of the respirator or deactivated the protective electrostatic charge on the mask filter. But Muratoglu’s group soon hit on decontaminating the masks with hydrogen peroxide vapor. That met all of the criteria for preserving the integrity of the masks and killing the virus.
Successfully decontaminating one mask in the lab, however, was a far cry from decontaminating the thousands used every day. In mid-March, the researchers found a defense contractor, Battelle, that had developed a hydrogen peroxide vapor system as part of its bioterrorism research, and Massachusetts state officials chose the company as the vendor for a decontamination facility that was set up in a former Kmart. Starting on April 7, 50 people there would sort, label, package and deliver thousands of decontaminated N95 respirators daily, making sure that individual clinicians received their original masks back. “N95 decontamination was a game-changer,” Raeke says.
That left the problem of a ventilator shortage. In severe cases, COVID-19 causes the air sacs in the lungs to fill with fluid, leaving patients gasping for air. Mechanical ventilation, in which a tube is inserted through the nose or mouth to push air into the lungs, may prevent further damage and restore oxygen to organs and tissues. But looking at the case projections in early February, it wasn’t clear there would be enough ventilators to go around.
All told, the hospital had 150 ventilators. Robert Kacmarek, director of respiratory care, was ultimately able to buy, rent or borrow an additional 100, which would prove to be more than enough to provide care for the peak number of patients on ventilators—188, on April 19. But there was one complication. The ventilators that Kacmarek added for the surge weren’t wired to the hospital’s central monitoring system, which sounds an alarm if a ventilator malfunctions or a patient needs help. The solution was decidedly low-tech. More than 100 MGH employees, mostly researchers whose labs were closed during the pandemic, took shifts outside patients’ rooms. They sat and listened, around the clock, for any problems the machines might encounter.

Source: Israel Vargas: Photos from MGH Photography
Even as MGH raced to prepare for a flood of infected patients, hundreds of people at the hospital threw themselves into a different kind of effort. The Massachusetts General Research Institute (MGRI) is the country’s largest research enterprise based at a hospital, and it normally oversees more than 1,900 simultaneous clinical trials, often collaborating with U.S. agencies, other research institutions and pharmaceutical companies. Now MGRI shifted its mammoth resources to investigate potential COVID-19 vaccines and therapies. “Research is in the DNA of what we do, and especially in a crisis like this, we knew we had to generate knowledge,” says Paul Biddinger, director of MGH’s Center for Disaster Medicine and chief of the MGH Division of Emergency Preparedness.
Researchers at the Ragon Institute were among the first to sound the alarm about the seriousness of what was to come. When the draft genome of the virus was released in early January, they noticed similarities to the genetic makeup of past coronaviruses as well as peculiar, worrying features. “It had the hallmarks of a virus that could spread substantially,” says Dan Barouch, a group leader at the Ragon and director of the Center for Virology and Vaccine Research at Beth Israel Deaconess Medical Center.
By Monday, January 13, after a frenzied weekend analyzing the genome, Barouch and his team had designed several prototypes for a human vaccine. They planned to test two delivery mechanisms. One would use DNA molecules to ferry in genetic materials to host cells; the other would use a deactivated common cold virus known as Ad26—adenovirus serotype 26. Either way, the genetic instructions from the vaccines would prompt the recipient’s cells to start producing the virus’s distinctive spike protein. This in turn would stimulate an immune response from the body, training it to make antibodies that could prevent further infection. Barouch had used the Ad26 virus approach in an experimental HIV vaccine and an experimental Zika vaccine, both of which are in human trials.
Animal studies in mice and monkeys began less than a month later. In a proof of concept study, monkeys were infected with COVID-19, then exposed to the virus a second time to see whether it was at all possible to generate a protective immune response against this virus. One reason it has been almost impossible to develop an HIV vaccine is that the virus doesn’t generate what is known as natural protective immunity—the human body, exposed to HIV or to some form of the virus in a vaccine, doesn’t develop antibodies that could fight off infection. Some scientists feared that COVID-19 might fall into the same category—“and if there was no sign of natural protective immunity, it was unlikely any vaccine would succeed,” Barouch says.
Working at home on an early April afternoon, he received an email with the first study results—which showed that monkeys exposed to the new vaccines did indeed develop natural protective immunity. “I was very nervous before I opened the attached file because I knew the numbers from our studies would have profound implications not just on our vaccine, but also on all vaccine efforts globally,” Barouch says. In May, the team released findings in two published studies, confirming that several versions of the prototype vaccine produced antibody responses in the test animals, preventing infection.
Meanwhile another team had also begun studying the COVID-19 genome that was posted online. Luk Vandenberghe, director of the Grousbeck Gene Therapy Center at Massachusetts Eye and Ear, and Wenlong Dai, a postdoctoral research fellow in Vandenberghe’s lab, had come up with their own vaccine candidates within a few days.
Central to their model is another kind of well-established vector. The adeno-associated virus, or AAV, is commonly found in humans, doesn’t cause disease and has been used successfully in experimental gene therapies, including in two drugs now approved by the Food and Drug Administration to treat rare diseases. In those therapies, AAV is efficient at transporting genetic material into cells, which could be an advantage in developing a vaccine that would operate in similar ways.
In this case, however, Vandenberghe was looking at a slightly different AAV—AAVrh32.33, a hybrid combining two AAVs found in monkeys. He had created it 15 years earlier, as a graduate student in a lab led by James Wilson, director of the Gene Therapy Program at the University of Pennsylvania’s Perelman School of Medicine, and it had shown early promise in HIV vaccines before ultimately being shelved. Now Vandenberghe wondered whether the rh32.33 hybrid might be a particularly effective delivery vehicle for fragments of the spike protein of COVID-19. Wilson endorsed the idea and eventually signed on as a collaborator, and by the end of February, 18 scientists in Vandenberghe’s lab were working on the vaccine project full time.
In early March, Vandenberghe began sharing data about the vaccine with Mason Freeman, director of MGH’s Translational Research Center. Freeman and his Center had previously participated in AAV gene therapy studies and he was familiar with aspects of Vandenberghe’s research. “I had never thought about AAV and vaccines,” Freeman says. “But based on how well AAV works in gene therapies, this seemed like a really exciting, unique approach.”
Freeman has led efforts to design clinical studies for AAVCOVID, the experimental vaccine, and in preclinical testing of two variations, it produced a robust immune response in mice and monkeys. Human trials are likely to follow, although first the FDA has to approve use of rh32.33, the engineered hybrid AAV vector, which doesn’t occur naturally and hasn’t yet been shown to be safe in humans. But if trials proceed and are successful, this vaccine might have an advantage over the dozens of others now under development. Only a miniscule amount would be needed for each dose, and one production run produces a million doses. “There are dozens of vaccines moving forward, but this one is eminently doable,” Vandenberghe says.

Source: Israel Vargas: Photos from Getty Images
On February 26, the biotech company Biogen welcomed about 175 executives from around the world to the Marriott Long Wharf hotel in Boston for a two-day conference. One week later, Biogen reported that two attendees who had flown home to Europe had tested positive for COVID-19—and on the same day, March 4, several local Biogen employees who had also attended the conference showed up at the MGH Emergency Department, asking to be tested for the virus.
The Biogen conference would later be recognized as one of the first major “superspreader” events in the United States, responsible for many of the COVID-19 infections treated at MGH. But tracking down which attendees might be infected required testing for the virus, which in early March was available only through the state public health laboratory. MGH had to ask the Massachusetts Department of Public Health for permission to perform any COVID-19 test—and based on the CDC criteria at the time, only three of the five employees qualified for testing. Two days later, MGH had the results—all three Biogen employees had COVID-19. Later that morning, Erica Shenoy of the MGH Infection Control Unit had a conference call with the Department of Public Health, counterparts at Brigham and Women’s Hospital and Biogen’s medical director. It was agreed that the two hospitals would test Biogen conference attendees identified by the company as well as symptomatic household members—a total of approximately 170 people.
That night—two days ahead of its planned opening date—MGH launched a testing site it had constructed in an indoor ambulance bay adjacent to the ED. Although the space, which normally accommodates seven ambulances, couldn’t be heated or cooled, it was spacious. Over the next three weeks, 2,667 people with COVID-19 symptoms were tested for the virus in the ambulance bay. “In March, we had no way of knowing that people without symptoms could be infected—the Department of Public Health had no capacity to test asymptomatic people,” says David Hooper, chief of the MGH Infection Control Unit and associate chief of the Division of Infectious Diseases.
The shortage of testing was the most dangerous blind spot in tracking the early spread of the pandemic. “Even getting approval to test patients with clear symptoms had become extremely challenging, because the case definition that dictated whether or not testing was permitted was so restrictive,” Shenoy says. So researchers at MGH became focused on inventing their own version of the test. The MGH pathology department’s microbiology lab and the Center for Integrated Diagnostics set about creating an assay that could separate the infected from the uninfected.
Any test would have to use CDC protocols governing testing chemicals and equipment, which led to a scramble to assemble the needed components. And the testing platform also had to match CDC targets for its ability to detect minimal amounts of COVID-19. The team tested its first assays against swab samples from Biogen employees who had tested positive for COVID-19. Then it used the test to analyze biosamples from former patients who had been hospitalized for respiratory problems before the pandemic. “We needed to be 100% sure our test was specifically detecting SARS-CoV-2 and not other common cold viruses,” says Jochen Lennerz, medical director of the MGH Center for Integrated Diagnostics.
In early March, just one week after work on the test began, it went live, and MGH became one of the first academic medical centers to gain FDA emergency use authorization for COVID-19 testing in the Boston area. The platform soon ramped up from 30 specimens a day to 150. It was another hurdle cleared, and with the testing, ventilator, mask and ICU conundrums on their way to solutions, the hospital was as prepared as it could be for the onslaught of COVID-19 patients to arrive. They were not long in coming.
Dossier
“Assessment of Community-Level Disparities in Coronavirus Disease 2019 (COVID-19) Infections and Deaths in Large US Metropolitan Areas,” by Samrachana Adhikari et al., JAMA Network Open, July 2020. Poor and marginalized communities faced the worst of the early pandemic, according to the research letter.
“The Association Between Symptoms and COVID-19 Test Results among Healthcare Workers,” by Chana Sacks et al., Annals of Surgery, Sept. 15, 2020. The study outlines how hospitals can keep workers safe with PPE and testing protocols.
MassGeneral.org/news/coronavirus/research. The page hosts the latest in diagnostics, treatments, trials and disease biology from researchers at the hospital.
Stay on the frontiers of medicine
Related Stories
- Chapter 2: The Virtues of Necessity
As the first COVID-19 patients arrived, pressure mounted to discover how the disease worked and how it could be beaten back.
- Chapter 3: The Fight That Lies Ahead
When the caseload began to ease, clinicians came to grips with the new normal as researchers set their sights on ending the pandemic for good.
- 200 Years of Preparation
Since its founding in 1811, MGH has both faced pandemics and learned from them.