Published On June 10, 2016
YOUR HEART WILL BEAT ABOUT 100,000 times today, dispatching oxygen-filled blood through vessels that branch into every part of your body. It’s an incredibly long and twisting haul. If you could take all of the arteries, veins and capillaries out of your body and lay them end to end, that one strand would reach around the globe—twice.
The current in these channels carries a tumbling mix of cells. Floppy, rust-colored red blood cells are packed with iron-rich hemoglobin proteins that give blood its color. Those proteins hold on to oxygen molecules, making each red blood cell a cargo hauler, a truck in an endless convoy that completes one loop through your body every 20 seconds. All along the route, red cells offload their oxygen, delivering this raw ingredient of energy to every cell, every tissue, every organ in your body.
Because the circulation of red blood cells is so routine—the ultimate milk run—those cells may seem unlikely vehicles for radical new medical treatments. Yet researchers and startups have recently taken an interest in how these cells can be nudged to treat various conditions. The experimenters see benefits in the cells’ structure, durability and ubiquity. As red cells follow their dependable route, the thinking goes, why not let them take some drug or other therapeutic agent along for the ride?
Samir Mitragotri, director of the Center for Bioengineering at the University of California, Santa Barbara, has dubbed this notion “cellular hitchhiking.” Blood is composed of a buffering liquid called plasma, as well as many kinds of circulating cells, including the platelets that help blood clots form, a variety of white blood cell types involved in immunity, and red blood cells. While all of these cells are being studied as drug-delivery agents, Mitragotri believes red cells have the most potential. “They circulate in the blood for a long time, and they have access to tissues throughout the body,” he says. “For a lot of therapeutics, that’s what you want.”
Researchers are now testing a range of techniques that would take advantage of the steady presence and go-everywhere access of red blood cells. Mitragotri’s lab is among those investigating ways to hook useful particles to the surface of red cells. Others want to tuck the medicine inside the cell. And one startup genetically alters the cell itself, causing it to produce a therapeutic agent. What all of the researchers have in common, Mitragotri says, is their interest in “hybrids” that mix manufactured medicine with the human body’s natural cells. Whether any of their approaches succeed may depend on the altered cells’ ability to slip into the convoy nearly unnoticed.
Red blood cells are remarkably elastic, able to change their shape as necessary. Their flexibility, required of cells that must squeeze through tiny blood vessels and then bounce back to their normal shape, allows researchers to use a nifty trick that makes use of osmosis. If a cell has a concentration of some dissolved substance (salt, for instance) that is higher than that of the fluid around it, water rushes into the cell to equalize the two concentrations. Pores on the red cells’ membranes open to let the water flow in—and when they do, a drug dissolved in the water can flow in too. Then the researchers can transfer those cells to a patient, and the drug catches a ride into the bloodstream inside the protective shell of the red cell.
Many researchers have seen the clinical potential in this encapsulation process, though few have gone beyond lab experiments. But Erytech Pharma, a French biotechnology company, has found a way to standardize the process for an industrial production line, churning out altered red cells that contain a treatment for leukemia and perhaps many other kinds of cancer.
Erytech’s first clinical trials enrolled patients with acute lymphoblastic leukemia (ALL), in which bone marrow produces cancerous cells that circulate through the bloodstream. Patients received transfusions of red cells carrying an encapsulated enzyme called asparaginase, a standard form of chemotherapy for ALL. Typically, though, asparaginase is injected into the bloodstream as a free-floating molecule, rather than as cargo protected inside red cells.
Asparaginase breaks down an amino acid called asparagine, which all cells need to grow and multiply. Normal cells both produce their own asparagine and also absorb some, the byproduct of digested food, which circulates in the blood. Cancer cells, however, can’t manufacture asparagine, and depend entirely on the circulating supply for their survival—and the enzyme asparaginase clears the bloodstream of that crucial amino acid. “The normal cells don’t suffer, because they can make their own asparagine, but the tumor cells starve,” says Erytech CEO Gil Beyen. If the treatment continues long enough, the cancer may disappear completely.
Asparaginase, in its free-floating form, can cause severe allergic reactions, so some leukemia patients can’t use it. The body also clears it from the bloodstream in just a day or so. Tucked inside red blood cells, though, the hidden asparaginase doesn’t trigger an allergic reaction, and instead of being quickly eliminated, the enzyme does its work for several weeks.
Erytech is testing its product on a second blood cancer, acute myeloid leukemia, and Beyen believes it might also have a chance to beat back solid tumors, with another trial studying its safety and efficacy in patients with pancreatic cancer. All tumor cells need asparagine to grow and multiply, so the same principle of action may hold true. Free-floating asparaginase has been tried as a treatment for pancreatic cancer, Beyen says, but toxicity was a problem.
With many of Erytech’s trials still in their early stages, it’s impossible to know how effective its product will be, particularly for treating solid tumors. But Paul Burke, founder of the biotech consulting firm Burke Bioventures, notes that many innovative drugs fail because “they’re not sufficiently compatible with biology.” Erytech’s strategy of pressing natural blood cells into service might be a way around that problem. “Rather than taking a synthetic approach, the starting point is the biological cell,” Burke says.
After a red cell has done its rounds for about 120 days, completing more than 500,000 laps around the body, the spleen and liver dismantle the worn-out cell and recycle its components. For example, hemoglobin breaks down into iron and other molecules, which are distributed to tissues throughout the body for reuse. During the demolition process, the spleen and liver present the reusable pieces to immune system cells that are always poised to destroy foreign objects, teaching them to recognize the pieces as safe instead of attacking them as alien threats. “We were interested in ways to exploit that biology,” says Jeffrey Hubbell, a professor of bioengineering at the University of Chicago and Switzerland’s École Polytechnique Fédérale de Lausanne.
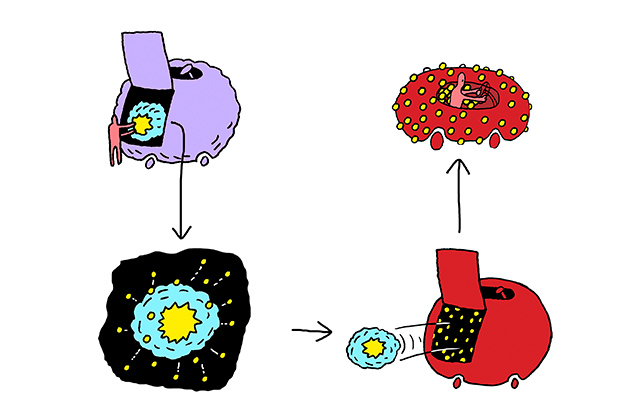
Hubbell’s immunotherapy research became the basis first of the Swiss startup Anokion, and then the Boston spinoff Kanyos Bio. “Our starting point was, gosh, red blood cells turn over at a rate of 1% per day,” Hubbell says. “That’s a whole lot of cells.” Indeed, about 200 billion red cells undergo natural cell death every day. Hubbell and his colleagues came up with the idea of making use of the red cell dismantling process, which the immune system considers so routine. They want to extend that ho-hum familiarity to proteins that otherwise cause problems in autoimmune disorders.
In celiac disease, for instance, gluten proteins found in wheat trigger an immune response in susceptible people. White blood cells in the digestive system go into attack mode, damaging the lining of the small intestine. Kanyos takes these problematic proteins and binds them to red blood cells, so they’ll be included in the recycling process when the cells are broken down. “As the red cells age, die and are cleared away, the protein will also be cleared,” Hubbell says. The immune cells see the whole package and recognize all of the components as harmless. In several mouse studies, he and his colleagues have proved that this idea can work. As the immune system naturally tolerates the red cell debris, it can also learn to tolerate the problematic protein.
The team is also interested in applying these lab findings to type 1 diabetes, another autoimmune disease in which the body, for unknown reasons, destroys its native insulin-producing cells. But this technique could be useful in many different immune system disorders, Hubbell says, from routine allergies to complex diseases such as multiple sclerosis. Theoretically, his system could work with any protein that triggers an immune response.
Hubbell’s current experiments aim to determine how long the immune system retains its “memory of tolerance” to the problem protein. His most recent study, published in 2015 in Scientific Reports, demonstrated that the therapy induced long-lived regulatory T cells, which provide tolerance’s memory. He says memory is important because “that determines how often you have to repeat the dose.”
Mitragotri of UC Santa Barbara, who isn’t affiliated with Kanyos, says the companies working on this frontier of medical research will need to answer a series of questions. “If you modify the red blood cell,” he asks, “how does the body respond to it?” If such products prove to be safe and effective, next comes the question of how the new treatments compare with existing therapies. “They have to establish uniqueness, and show what they can do that other approaches cannot,” he says. Still, Mitragotri is optimistic that hacked red blood cells will eventually make it to the clinic. “I think they will be able to do things that routine therapeutics can’t do,” he says.
Perhaps the most out-there idea for modifying a red blood cell starts with a unique detail of the cell’s interior. As a young red cell matures, it ejects its bulky nucleus to take on the characteristic floppy form that will allow it to squeeze through tiny capillaries. Red blood cells are just about the only cells in the body that don’t have nuclei.
Rubius Therapeutics, a Boston startup, begins with hematopoietic stem cells, which have the potential to grow into any kind of blood cell in the body. The company inserts DNA into the stem cells’ nuclei, giving them genetic instructions for manufacturing a useful therapeutic protein. Then Rubius guides the stem cells in culture along the developmental pathway that turns them into red blood cells. During the initial phase, when the stem cells have become “progenitor” red cells, their altered DNA instructs them to mass-produce the therapeutic protein. In the next phase, the cells eject their nuclei that carry the modified DNA. The mature red cells can then be infused into the patient’s bloodstream—with the therapeutic proteins still inside or on the surface.
This technique may allow Rubius to avoid some of the issues that come with the medical use of genetically modified products. Because these modified cells don’t have nuclei when they enter the bloodstream, they can’t divide and replicate themselves to spread cells with altered DNA through the body, which could have unknown consequences.
Using this process, the company can go from a raw idea to a prototype drug in three weeks, says Rubius co-founder and CEO Avak Kahvejian. “We can take the stem cell, drop in the gene, move the cell through the differentiation pathway and end up with modified cells that have the biotherapeutic inside,” he says.
His first clinical target is a metabolic disorder called phenylketonuria (PKU), in which people lack an enzyme needed to break down an amino acid called phenylalanine that becomes toxic when it builds up in the bloodstream. Babies are tested for this genetic disorder at birth, but there is currently no cure, and treatment focuses on carefully controlling the diet to limit intake of the amino acid—found in common foods including meat, fish, beans and nuts. If PKU is untreated or poorly managed in infants, there can be brain damage. People with PKU must adhere to the strict diet throughout their lives or risk neurological problems.
The Rubius solution is to send altered red cells that act as tiny “bioreactors” into the bloodstream. Thanks to the earlier genetic modification, these cells will be stocked with the missing enzyme needed to break down phenylalanine. As the red cells circulate through blood vessels they’ll naturally draw in any phenylalanine they encounter, and will do the job of chopping it up into safe components. “That will allow patients to process phenylalanine when they eat it, without having to take a medicine every day,” Kahvejian says. As red cells typically last for 120 days in the body, these “bio-reactor” cells could theoretically manage incoming phenylalanine for months before a new dose is required.
Consultant Paul Burke, who advises biotech startups, calls this and other research on cellular hitchhiking creative, exciting—and likely to face a thousand setbacks before any great success, just as almost all promising new drugs do in the development process. “There’s room to make a million mistakes; there are things that no one can foresee,” he says.
But Burke also notes that such bold research efforts are the provenance of breakthroughs. “There are a lot of serious unmet needs in medicine,” he says, including conditions targeted by the red blood cell pioneers. “The only way we get ahead is if researchers and companies are willing to step out and try something different,” he says. Now we’ll see what happens when they step out and stick out their thumbs.
Dossier
VIDEO: “The Erythrocyte Life Cycle,” Medic Tutorials. This 10-minute video summarizes the processes involved in red blood cell formation, circulation and breakdown.
“Cell-Mediated Delivery of Nanoparticles,” by Aaron C. Anselmo and Samir Mitragotri, Journal of Controlled Release, Sept. 28, 2014. The authors review a variety of ideas for using circulating blood cells for drug delivery.
“In-Vitro Stem Cell Derived Red Blood Cells for Transfusion,” by Hyun Ok Kim, Yonsei Medical Journal, March 2014. Some researchers are trying to mimic the natural production of red blood cells to create supplies of synthetic blood. Kim reviews the progress and remaining challenges in this endeavor.
Stay on the frontiers of medicine
Related Stories
- The Bleeding Edge
A Boston lab looks to the plucky and omnipresent red blood cell for a new generation of therapies.
- Eating Away at You
Avoid gluten, and celiac disease loses its sting. But research continues, and breakthroughs might treat other disorders too.