Published On June 10, 2016
REUBEN HILL, 22 years old, a doctoral student in physics and a judo aficionado, had a tumor the size of a golf ball in his brain. The mass was next to the brain region that governed Hill’s ability to speak and sing—he was in a choir, too. In the operating room at London’s Charing Cross Hospital, neurosurgeon Babar Vaqas realized the challenge he faced in removing the tumor. Take too little tissue, and it could continue to grow and worsen the young man’s prospects. But take just a few millimeters too much and his patient could be permanently silenced. “Your margin of error,” Vaqas said afterward, “is very, very small indeed.”
Adding to the difficulty, brain tumors arise from normal cerebral matter, making it difficult to identify where healthy tissue ends and abnormal tissue begins. To aid in that task during Hill’s operation in August 2015, Vaqas—who conducts research at London’s Imperial College—used a specially designed handheld laser to map the tumor’s location. As he moved the probe around, it didn’t cut tissue, but delivered second-by-second information about the light reflecting off of the brain to a spectroscope, a device that translated the distinctive “signatures” produced by malignant and healthy tissue, respectively, on a monitor, which helped guide Vaqas’s surgical forceps. When Vaqas was ready to remove the tumor, a speech therapist awoke Hill and asked him to speak, then to sing, to ensure that no functioning brain cells were being damaged. BBC reporter Fergus Walsh observed the operation and reported, “With the lights dimmed, Mr. Hill sang these poignant words from the hymn ‘10,000 Reasons’: ‘Whatever may pass and whatever lies before me, I’ll still be singing when the evening comes.’”
Lab tests determined that the tumor wasn’t malignant, and Hill—who himself studies laser technology—made a full recovery. This use of lasers in brain surgery made headlines, following earlier work by a team in Montreal that had used laser-based technology to help remove brain tumors.
Lasers themselves aren’t new to cancer treatment. Whereas normal white light is made up of all of the colors of the visible spectrum, each of which travels at a particular wavelength, a laser produces light at one specific wavelength and travels as a narrow, pinpointed beam. That precise focus and lasers’ thermal properties have long made them useful for destroying malignant tissue, and today they’re used to treat various forms of cancer. The most common approach, called laser ablation (or removal), uses the heat of the fixed light beam to kill cancer cells.
But now the notion of employing lasers and other forms of light to analyze tissue properties and help guide the removal of tumors is quickly gaining interest among surgeons and scientists.
“This area is ready to explode,” says head and neck surgeon Eben Rosenthal, medical director of the Stanford Cancer Center, who reviewed the growing field of optical- and fluorescence-guided oncologic surgery for Annals of Surgery last year, and who has studied the use of dyes attached to cancer drugs as a way to light up tumors. Within a decade, Rosenthal predicts, light-guided removal of tumors will be standard operating procedure.
Meanwhile, other researchers are exploring new ways to apply an idea that has been around for nearly half a century. They are using light to turn on drugs that destroy tumor cells from within. Photodynamic therapy (PDT) was first used decades ago, but now doctors at several institutions are exploring how to deploy the treatment against various cancers. Still others are considering how light could be used to trigger the delivery of payloads of chemotherapy inside tumors themselves, thus sparing nearby healthy cells damage from toxic cancer drugs.
Doctors have been removing cancerous tumors surgically at least since the days of ancient Egypt, and the introduction of anesthesia and antiseptics to prevent infection in the 19th century made oncological surgery more common. But the basic approach has remained the same—to cut out not only the tumor but also a border of healthy tissue, known as a margin. If subsequent lab tests find no cancer cells in the margin, the assumption is that all of the tumor was excised. If a margin tests positive for cancer cells, then the procedure has failed to eradicate all of the tumor, which could continue growing.
X-rays, magnetic resonance imaging (MRI) and computed tomography have made it easier to locate and estimate the size of tumors prior to surgery. Yet because tissue can shift, pre-operative imaging doesn’t help much when neurosurgeons start to cut. And while they can use MRI during a procedure to provide real-time images of the tumor and surrounding tissue, that process is time-consuming and cumbersome. As a result, surgeons today rely mostly on the same tools they used a century ago—their vision and touch. “We use very gross measures to identify cancer” during surgery, says Rosenthal.
But malignancies often elude those human tools. A tumor in the breast, for example, may be firm to the touch and create an image on mammography, but often small clusters of cells that break off from the primary mass won’t be palpable or visible, says chemical biologist Matthew Bogyo, also from Stanford. That’s why a margin of presumably healthy tissue has to come out, too. Often enough, however, when that sample is sent to a pathology lab and studied under a microscope, cancerous cells remain, likely leading to another operation.
Light already plays a vital role in detecting cancer, if not also in removing it. Standard white light provides illumination for endoscopy procedures, for example, so that cameras attached to endoscopes snaked through the digestive tract can detect polyps and other lesions. Flat polyps in the colon and elsewhere in the digestive tract, however, can be just as deadly as raised ones, and they often evade detection with endoscopy. “You just miss them,” says Bogyo.
Other uses of other kinds of light may be more helpful in illuminating cancerous tissue. Fluorescence, for example, occurs when an object or substance gives off radiation, usually in the form of visible light, upon exposure to an external light source or another type of radiation. And a few fluorescent dyes are currently employed in surgery. Injected or swallowed, they cause certain tissues to glow when exposed to a particular wavelength of light; cameras capture the fluorescence and depict it on monitors. Indocyanine green (ICG), for example, has been available for 60 years, but tends to remain in blood vessels instead of accumulating in tumors (in fact, some won’t absorb it), so it’s used mostly to track circulation during surgery—for example, to make sure a transplanted organ is getting enough blood.
Another fluorescent dye, 5-aminolevulinic acid (5-ALA), is approved in Europe (and used experimentally in the United States and elsewhere) for help in excising gliomas, an aggressive kind of brain tumor. A patient swallows a solution or a capsule containing 5-ALA, and cancer cells that absorb the dye glow a pinkish hue when exposed to blue light, providing a visual aid to surgeons. Still, some surgeons who have used 5-ALA say it isn’t selective enough. “In our experience, it lights up more than just the tumor that needs to be removed,” says Vaqas.
The trick, then, is to get fluorescent dyes into tumors and only tumors, so that the borders of an image of a malignant mass are crisply defined. To make that happen, Rosenthal and his colleagues started with the drug cetuximab, an antibody that binds to a protein called EGFR that’s present in more than 90% of head and neck cancers. The researchers united cetuximab with a dye called IRDye800. In 2015, following a trial involving a dozen patients with squamous cell carcinomas of the head or neck, Rosenthal’s group published the first study demonstrating that an antibody-fluorescent dye combination could effectively delineate a tumor from healthy tissue. “We can see cancer in real time,” says Rosenthal. The dye also illuminated near-microscopic cancers not yet large enough to be detected by other means.
In another first, researchers at Memorial Sloan Kettering Cancer Center in New York City injected glowing nanoparticles known as C-dots (so named because they were developed at Cornell University) into patients with melanoma that had metastasized throughout their bodies. A nanoparticle is essentially a machine on a microscopic scale—in this study, 6 to 7 nanometers in diameter. (One nanometer is equal to one billionth of a meter.) C-dots are embedded with fluorescent dye tagged with a substance that binds them to cancer cells, as well as with radioactive iodine, which makes the nanoparticles visible on a positron emission tomography scan. This trial was designed to test how C-dots diffuse in the body—they are excreted within a day or so and appear to be nontoxic—but they have already been used to illuminate metastatic melanoma in pigs, and, in clinical trials, researchers are already seeing whether C-dots can serve the same purpose in people, mapping the spread of their cancer, according to radiologist Michelle Bradbury, who is overseeing further clinical trials of these and other fluorescent molecular “probes” at Sloan Kettering.
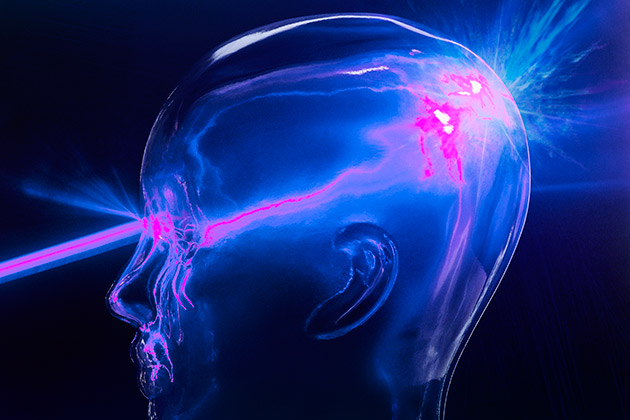
Fluorescent dyes are one way that light can help define tumors. But a new use of laser technology can also search tumors out, utilizing the reflective properties of the tissue. All matter absorbs and reflects light, but each kind of material does that in a unique way, in patterns that can be measured and characterized using spectroscopy. And while most light bounces off objects unchanged, a small amount undergoes a shift in energy, caused by the particular pattern of vibration of molecules in whatever material that light encounters.
Those properties of light produce the signature that can be used to distinguish healthy flesh from abnormal tissue. The laser device used to remove Reuben Hill’s brain tumor collects reflected light, the patterns of which are interpreted by a method called Raman spectroscopy.
That laser is a modified version of a device called the Aura, manufactured by Verisante, a Canadian company. Scientists at the British Columbia Cancer Agency created the technology, initially focusing on skin cancer, with the intention of extending it to major cancers like colon and lung. In one second, the handheld probe can distinguish between melanoma and a harmless skin mole with 95% accuracy, theoretically allowing a doctor to decide during an office exam whether to proceed with a biopsy of a particular lesion. (The Aura is approved for use in Canada and several other countries, and Verisante is seeking U.S. Food and Drug Administration approval to market the device in the United States.) Verisante has also adapted the technology for use in an endoscope, which could help diagnose lung, esophageal and colon cancers.
Neurosurgeon Kevin Petrecca and his colleagues at the Montreal Neurological Institute and Hospital have developed their own distinct tumor-detecting device that also happens to use Raman spectroscopy, which they have tested in more than 80 patients who have glioblastomas or other brain tumors. Petrecca says their device identifies malignant cells with 97% accuracy, finding cancer cells that don’t show up on MRI scans. Now, in preparing to apply for FDA approval, his group is conducting a clinical trial to demonstrate whether the tool can help surgeons by revealing more of a tumor than can be seen through other methods.
Still another way to deploy light against cancer mounts a multi-pronged attack using a light source, a drug that makes cells sensitive to that light, and oxygen. Photodynamic therapy was first tried early in the 20th century as a treatment for skin cancer and other conditions. A later version of PDT, still used today, was developed in the 1970s at Roswell Park Cancer Institute in Buffalo, N.Y.
A day or two before undergoing PDT, a patient normally will receive an injection or topical application of a photosensitizer, a nontoxic drug that travels through the bloodstream and is absorbed by cells. The interval allows tumor cells to take in as much of the drug as possible. Light emitted from a laser aimed at the tumor activates the photosensitizer, which interacts with oxygen in the tissue to generate reactive oxygen molecules—often known as free radicals—that instantly destroy tumor cells. The blood vessels that nourish the tumor are shut down by collateral damage from the PDT, and the body responds by rallying immune cells to mount an attack on the tumor site. Because the drug is laser-targeted and activated only inside malignant cells, PDT causes few side effects beyond brief pain and inflammation (though patients must avoid direct sunlight for several weeks).
On its own, PDT can cure some small, early-stage cancers, offering patients an alternative to radiation or surgery, which tend to have much greater side effects. The most widely used photosensitizer, Photofrin (porfimer sodium), is FDA-approved for treating specific lung and esophageal cancers, as well as Barrett’s esophagus, a precancerous condition. But denser, bulkier tumors are hard for light to penetrate, and that limits the use of PDT as a stand-alone therapy. It can supplement standard care, however, and Roswell Park and other cancer centers use Photofrin off label with PDT on a variety of malignancies.
Radiation oncologist Charles Simone of the Perelman School of Medicine at the University of Pennsylvania says that in their experience, combining PDT with surgery has dramatically improved the survival of those with mesothelioma, a malignancy that forms in the lining of the lungs, often in people exposed to asbestos. “The standard of care,” says Simone, “had been to take out the entire lung”—an approach that can severely limit patients’ quality of life by reducing their lung function and as a result their ability to perform even basic daily tasks.
In a treatment Simone and his colleagues at Penn have pioneered, surgeons remove only the lung’s lining, or pleura, and then apply PDT to kill microscopic cancer cells that may be left behind. To make the treatment more effective, they fill patients’ chest cavities with saline fluid infused with fats that help disperse the laser’s red light more fully through the cavity. “It’s almost like a disco ball,” says Simone. In a 2012 Penn study, people with epithelioid mesothelioma who had the PDT-enhanced treatment lived about two years longer than is usual for patients who receive chemotherapy alone—an improvement that provided the longest survival ever recorded for mesothelioma patients.

Like many other cancer therapies, however, PDT can trigger survival mechanisms in a tumor that allow malignant cells to spread throughout the body. To overcome that problem, researchers at Massachusetts General Hospital’s Wellman Center for Photomedicine created a dual-function nanoparticle construct. It contains the photosensitizer benzoporphyrin derivative (BPD) in a membrane enclosing a polymer nanoparticle filled with the cancer drug cabozantinib, which can help deter growth of cells and their migration from the tumor, and shut down the vascular pathways that support tumors.
Cabozantinib is FDA-approved for treating thyroid cancer, but its toxicity limits the amount a patient can receive. The MGH team injected the combination nanoparticles into mice that had human cancer cells implanted to form tumors in their pancreases. The particles spread throughout the body, but the toxic drug, protected in the membrane, didn’t harm healthy tissue. Shining an infrared light (using optical fibers) on tumors activated the BPD and broke through the membrane, releasing the cabozantinib. The combination nanoparticle—which enhances normal PDT by releasing a chemotherapy treatment rather than depending solely on free radicals and also works on the vascular pathways that support tumors—shrank tumors more effectively than either standard PDT or cabozantinib used by itself, with little toxicity. And it did so with a dose of cabozantinib 1,000 times smaller than usual.
Meanwhile, researchers at UCLA’s Jonsson Comprehensive Cancer Center have developed nanoparticles with pores that can be filled with the molecules of a chemotherapy drug such as doxorubicin. Chemist Jeffrey I. Zink and his UCLA colleagues Fuyu Tamanoi and André Nel have designed nanoparticles that lock into and then are absorbed by cancer cells. In lab studies, a beam of infrared light shone on the tumor breaks chemical bonds in the nanoparticle, which releases the drug and kills the tumor.
“We can do this with almost any kind of cancer cell,” says Zink, who is investigating additional ways to coax nanoparticles into dropping a payload of medicine. But compared with other triggers, light has special advantages. “You can really pinpoint it, so that you avoid off-target effects, like hair loss or liver damage,” he says.
Isaac Newton, at the dawn of the scientific era, was fascinated by the possibilities of light. Centuries later, lasers and technologies are making good on that promise with tools that reveal what the eye can’t see otherwise, and treatments that can be delivered with microscopic precision. In cancer research, they promise to shed more light—literally—on one of the great remaining clinical enigmas.
Dossier
“The Status of Contemporary Image-Guided Modalities in Oncologic Surgery,” by Eben L. Rosenthal, et al., Annals of Surgery, January 2015. An overview of efforts to “light up” tumors using existing tools and new technologies.
“Nanotechnology-Based Drug Delivery Systems for Photodynamic Therapy of Cancer: A Review,” by Giovana Maria Fioramonti Calixto, et al., Molecules, March 2016. A look at how nanotechnology could make photodynamic therapy an even more potent weapon against cancer.
VIDEO: “Removing Margins of Error: Using Advanced Measurements to Push the Limits of Brain Surgery.” A 2015 review of light-based surgery by Babar Vaqas.
Stay on the frontiers of medicine
Related Stories
- Blue Light Special
Drug-resistant bugs have spurred research into a promising—and surprisingly simple—treatment.
- Age of Enlightenment
Light-activated genes, now illuminating brain circuitry in rodents and monkeys, may help solve mysteries of human disease.
- Killing Me Brightly
UV light proves itself as an annihilator of germs.