Published On May 3, 2007
“YOU’LL JUST HAVE TO LIVE WITH IT.” That’s what doctors told Donna Colicchio about her recurring attacks of atrial fibrillation. But after 20 years, Colicchio felt the scope of her existence shrinking. Once, when taking her niece to a movie, she dropped to her knees outside the theater and couldn’t get up. Another time, she brought a table set for Christmas dinner crashing down when she collapsed in her Groton, Mass., home. She had an attack on a transatlantic flight and on a beach in Barbados.
Colicchio was what physicians refer to as a “lone fibber,” an otherwise healthy person with episodic atrial fibrillation, the most common kind of heart arrhythmia, a condition characterized by irregular heartbeats. More than 2 million Americans have AF, and many, like Colicchio, have no other heart condition, though they all have an elevated risk of stroke.
In fact, by the early 1990s, Colicchio could have been cured in one fell swoop. All she needed was to have someone saw through her breastbone, pry apart her ribs, put her on a heart-lung pump, stop her heart from beating, cut a maze of incisions through her heart’s atrial chambers and sew her back up.
But Colicchio, like most lone fibbers, wasn’t considered sick enough to justify the risks of open-heart surgery. Most people with AF either aren’t treated at all or are cared for with drugs alone, even though medication relieves symptoms only half the time and may cause serious side effects. Drugs didn’t help Colicchio, but finally in 2003, with the attacks continuing, her doctor told her there was another alternative. She underwent a new catheter-based, or percutaneous (through the skin, implying a puncture rather than an incision), procedure that, like angioplasty, approaches the heart via a peripheral blood vessel. Though she required a follow-up treatment, necessary in about half the cases, she has been symptom-free ever since.
Such technological advances are helping a growing number of heart patients—from those who want to avoid or are unwilling to undergo surgery, like Colicchio, to others who are too sick or elderly to withstand the trauma of an open operation. The progress is good news for many patients, but what does it say about the future of traditional heart surgeons?
Already, surgeons have lost a third of potential patients for coronary bypass, the mainstay of cardiac surgery, to nonsurgical techniques involving angioplasty and drug-eluting stents. (That parallels developments in other specialties, in which open surgical operations for, say, removing a gall bladder, have given way to much less invasive video-guided endoscopic procedures.) Patients benefit from lower risks, less pain and horter hospital stays and recovery times. But many of the new procedures for AF and valve disease may be performed not by surgeons, but by interventional cardiologists and electrophysiologists, who are cardiologists specializing in arrhythmias.
The trend toward nonsurgical interventions (coupled with a profound decrease in reimbursement) has dulled the luster of cardiac surgery as a profession. As recently as 1990, hospitals could choose from among the crème de la crème of medical students to fill their cardiac surgery residencies. But in 2006 there were just 100 applicants for the 139 cardiothoracic surgical residency positions in the United States, and 28 of those applying were graduates of foreign medical schools. Some policy experts wonder whether even those numbers might be too high. But others worry that we could soon find ourselves with a critical shortage of heart surgeons to treat an aging population.
IN 1987, WHEN JAMES L. COX, A CARDIAC SURGEON AT the Washington University School of Medicine in St. Louis, pioneered the open-heart procedure that could have cured Colicchio, this much was known about the underlying physiology of AF. Normally, when the heart beats, an electrical signal known as a P wave arises from specialized muscle tissue in the heart’s atrial chamber and spreads out through a specialized conduction system, causing the heart muscle to contract. The heart then rests, waiting to be recharged for another cycle. With AF, an errant current commandeers the heart during that recharge period, causing a premature contraction and an irregular, less effective heartbeat.
Using computerized electrophysiology, which involves placing electrodes on the heart muscle, Cox mapped the cardiac rhythms of AF patients undergoing surgery for other disorders and discovered an order to the erratic rhythms. He was then able to design a pattern of crisscrossing incisions to create scar tissue that would interrupt those currents, a procedure that became known as the Cox-Maze. (Later, Cox and others found ways to accomplish the same goal without cutting the heart, using extreme cold, or cryosurgery; radiofrequency energy; microwaves; lasers; and, most recently, focused ultrasound to selectively destroy the tissue—all methods called ablation.)
In 1998 scientists in France made a discovery about the underlying physiology of AF that opened the door to simpler, ultimately less invasive procedures. The French team demonstrated that the major triggers for AF’s erratic pulses don’t originate from within the heart muscle, but rather, for reasons still unknown, from the pulmonary veins entering the back of the heart. Electrically isolating those triggers in the pulmonary veins—a procedure called pulmonary vein isolation, or PVI—effectively stops many forms of AF.
Because the pulmonary veins could be reached by catheters passed through peripheral veins, that discovery potentially eliminated the need to open up the chest to operate on the heart. In addition to percutaneous approaches, minimally invasive surgical procedures have been developed to create the maze lesions. Since 2003, several surgeons, including Randall Wolf at the University of Cincinnati and Michael Argenziano at the New York-Presbyterian Hospital/Columbia University Medical Center, have collectively performed more than 1,000 such procedures, in which they get at the heart through small incisions between the ribs (on each side of the chest) and isolate the pulmonary veins while the heart continues to beat.
But PVI can also be done even less invasively from inside the heart, approached via a percutaneous catheter. These so-called catheter ablations are handled by electrophysiologists such as Jeremy Ruskin, director of the Cardiac Arrhythmia Service at the Massachusetts General Hospital (MGH), who supervised Colicchio’s treatment in 2003. According to Ruskin, the field has grown markedly since then, and 10,000 to 12,000 patients now get catheter ablation PVI procedures each year.
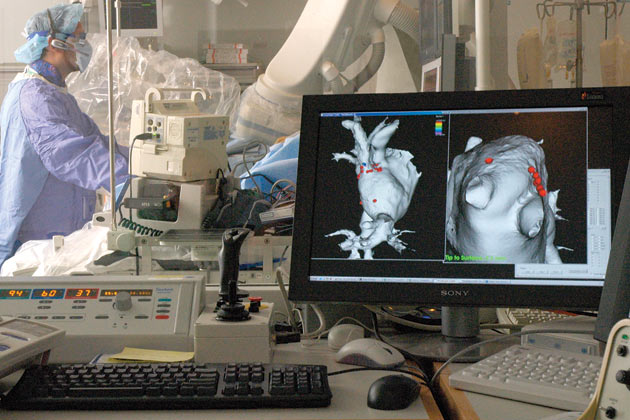
IN A RECENT PVI IN THE MGH’S ELECTROPHYSIOLOGY LAB, a patient lay under conscious sedation with a tangle of catheters inside her heart. One catheter, equipped with an ultrasound camera, provided an image of the heart’s interior and blood vessels. Another mapped the junction where the pulmonary veins entered the left atrium so that physician Vivek Reddy could maneuver the long, flexible ablation catheter to precisely the right points. On a monitor, those points appeared as wide ellipses of dots superimposed around each of the four pulmonary veins on a 3-D image of the patient’s heart.
Reddy asked the engineers in the control room to rotate the map to locate an ablation site and to “slice” the image open so he could examine the ridges and funnels where the pulmonary veins enter the heart. He would use that information to avoid any injury to the veins or the esophagus, which lies directly behind the heart’s left atrium.
Reddy guided the ablation catheter, cooled by saline flowing through the small electrode at its tip, toward one of the dots and delivered a burst of radiofrequency energy to ablate that site. After the team checked to make sure the electrical signals coming from there had been adequately reduced or eliminated, it was on to the next dot, and the next. This had to be repeated at all of the ablation sites for each of the four veins. The meticulous process, with nary a dramatic moment, went on for hours. (A new device using a balloon catheter promises to isolate an entire vein with just a few applications. It’s being tested in trials at the MGH and other medical centers.)
Catheter ablations appear to be slightly less effective than minimally invasive PVI surgery, and PVI surgery as a whole seems to be 10% to 20% less efficient than the open maze. But the PVI approaches enable many more patients to get help when drugs don’t relieve AF symptoms. The recommendations of the American College of Cardiology and American Heart Association Task Force on Practice Guidelines call for patients to first try drug treatment, then catheter ablation.
THE MILLIONS OF OLDER AMERICANS WITH another common heart ailment, mitral valve disease, normally must work their way up a similar decision tree. The most common form of this disease is mitral valve prolapse, and about 7% of people older than 65 have moderate to severe prolapse, which may cause serious health problems. During an eight-month period, 76-year-old Ruby Lord, of Smyrna, Ga., had gone from being able to mow her lawn to needing help caring for herself, a result of the failure of her floppy mitral valve. Her overworked, inefficient heart left her vulnerable to pulmonary congestion and atrial fibrillation.
Depending on the severity of their disease, patients with mitral valve prolapse can choose from a range of treatments, both medical and surgical. Typically, one of the valve’s two leaflets has stretched and no longer snaps shut, allowing blood to regurgitate into the left atrium and back into the lungs. In most of the approximately 30,000 surgical cases each year in the United States, a section of the leaflet is cut out to tighten the valve. In Lord’s case, her doctors told her that she’d likely survive an open-heart procedure, but that she would face significant risks and a long recovery. So she opted for an experimental procedure, the percutaneous installation of a MitraClip.
The MitraClip builds on the work of Ottavio Alfieri, a cardiac surgeon in Milan who in the mid-1990s first proposed what became known as the Alfieri stitch. Alfieri suggested that instead of cutting out part of the valve, a surgeon could tack together the valve’s two leaflets so that they’re pulled tight when the valve is closed and are still able to let blood flow through “bow-tie” orifices on either side of the stitch when the valve opens. Mehmet Oz, a cardiac surgeon at Columbia University’s College of Physicians and Surgeons, who saw Alfieri present this technique at an Italian conference in 1996, later helped design the MitraClip for doing the procedure percutaneously.
Now in a Phase II trial comparing it with open-heart surgery, the MitraClip was brand new in April 2005, when Peter Block, an interventional cardiologist at Emory Healthcare in Atlanta, snaked it up Lord’s femoral vein. Block steered a catheter into her right atrium, through the septum to the left atrium and down to the valve. Inside that catheter was another bearing the MitraClip, which is made of nitinol, a metal that can be bent and will automatically reassume its shape when released.
“The trick is lining up and delivering the catheter so the clip comes at the right angle to all three planes of the valve,” Block says. He opened the clip, advanced it through the valve and retracted it to capture both leaflets, then checked a color Doppler echocardiogram to see how much regurgitation remained. Though Lord had some residual regurgitation, her health improved markedly. “I’m as good as ever,” she says.
The MitraClip is one of several devices being tested as low-risk (though technically challenging) percutaneous fixes for mitral valve prolapse and other mitral valve problems. While Oz doesn’t expect percutaneous devices to have the effectiveness of surgical measures for every type of mitral valve disease, he does think they could each year benefit as many as 10,000 patients who do not want to accept the risks associated with open surgery or are in the early stages of the disease.
Percutaneous repairs for deteriorated aortic valves, though still a few years away, could ultimately provide similar benefits to a different, sicker group of patients. Aortic valve disease accounts for more than half of the approximately 100,000 patients who have valve surgery each year, and it’s a debilitating condition. “With symptomatic aortic stenosis, patients go steeply downhill,” says Igor Palacios, an interventional cardiologist at the MGH. “They have a 50% mortality rate in the first year, but surgery brings their life expectancy back to normal.” Very sick patients, however, can’t risk surgery, and while no percutaneous aortic valve device has yet been approved by the U.S. Food and Drug Administration, that could change soon.
Several devices—made by CoreValve, Edwards LifeSciences, Direct Flow Medical, Sadra Medical, Heart Technologies and AorTx—are in early trials. “And on the horizon are a host of extraordinarily clever aortic valve devices that are smaller, more user-friendly and easier to deploy,” says Emory’s Block. “They will also produce better long-term results.”
IF THE NEW AORTIC VALVE DEVICES PROVE as safe and effective as Block and others expect, they could drive another mainstay of cardiac surgery out of the operating room and into the cath labs. Retired surgeon James L. Cox, of Cox-Maze fame, doesn’t deny that the landscape is changing. “If you looked only at what has served as the basis of our profession, cardiac bypass surgery, there would be reason to worry,” Cox says. “And if our future depended on the development of more surgery for valve disease, we’d be in trouble.”
But Cox says doom and gloom about the future of heart surgery is nothing new. “They were predicting our demise before we even started,” Cox says. “I was an intern at Duke looking down the barrel of 10 more years of training when my mentor told us we’d have nothing left to do. I thought, ‘Holy cow! I might as well try to be a baseball player.’ But it has always turned out that just when you get to where it seems you might not have anything new to do, something pops up.”
That “something” might be what the MGH’s Palacios calls percutaneous valve surgery. He and other cardiologists believe that the trend toward less invasive cardiac procedures is creating new opportunities for surgeons and interventional cardiologists to work together to benefit different patient populations, such as those who have severe vascular disease that prevents the threading of a catheter from the groin to the heart. In one new technique a surgeon makes a small incision through the chest wall to reach the apex of the heart, and an interventional cardiologist inserts a very short catheter through the apex to the left ventricle to deploy an artificial aortic valve. Palacios dreams of the day when such collaborations become commonplace.
And even surgeons working on their own may find plenty to do, says Cox, who predicts that developing less invasive surgical procedures for atrial fibrillation and new devices to treat the nearly 5 million people with heart failure could ultimately triple the number of cardiac operations. Meanwhile, factors such as the obesity epidemic and the surge of baby boomers reaching the age of diabetes and cardiovascular disease could keep old-fashioned cardiac surgery in demand.
“If the current workforce of heart surgeons drops and if the number of cases suddenly increases, we will have a crisis,” says Cox. “Not the crisis of surgeons not having anything to do, but of not having enough surgeons to do what needs to be done.”
Dossier
“Percutaneous Heart Valve Replacement: Enthusiasm Tempered,” by R. David Fish, Circulation, October 2004. An insightful overview of the challenges facing surgeons and cardiologists in addressing the unmet need for less invasive means of treating valvular disease.
“Surgical Management of Atrial Fibrillation,” by James L. Cox,Medscape Cardiology, Vol. 9, No. 1, 2005. A concise overview of atrial fibrillation and the development of the surgical Cox-Maze, “mini-maze” and pulmonary vein isolation procedures for treating AF.
“Controversies in Cardiovascular Medicine: Should atrial fibrillation ablation be considered first-line therapy for some patients?” pro position by Atul Verma and Andrea Natale; con position by Benzy J. Padanilam and Eric N. Prystowsky,Circulation, Vol. 112, No. 8, 2005. An in-depth debate about the merits of catheter ablation to treat atrial fibrillation, compared with drug therapy alone.
Stay on the frontiers of medicine