Published On September 22, 2005
CONSIDERING THE PLIGHT OF MOST PEOPLE in her situation, Becky Hemingway was faring well. Her right side had gone numb, and she couldn’t speak. But her family had immediately suspected a stroke, and emergency room physicians at a hospital in South-bridge, Mass., not only made the diagnosis but also rushed the 31-year-old new mother to a regional stroke center in Boston, two hours to the east, where she could receive specialized care.
In contrast, the average stroke victim doesn’t get treated for almost a day, though the only approved current drug therapy must begin within three hours. Nine out of 10 strokes are ischemic, resulting from blood clots that cut off the oxygen supply to brain tissue, and the drug—tissue plasminogen activator, or TPA—dissolves clots. Given too late, however, TPA can further weaken blood vessels in the brain, increasing the risk of a potentially fatal hemorrhage. What’s worse, medical researchers have recently concluded that TPA itself might be toxic to some brain cells. Because of such risks, most physicians are reluctant to administer TPA even within the three-hour window. And the 3% of patients who receive the drug on time have a less than even chance of a positive outcome.
So stroke remains a prodigious killer, taking 165,000 lives annually in the U.S. (Only heart attacks and cancer are deadlier.) Another 535,000 stroke victims survive each year, but many have lifelong disabilities—ranging from complete paralysis and cognitive dysfunction to impaired speech. That makes stroke one of the nation’s most expensive diseases, costing an estimated $57 billion a year.
With a large clot obstructing her middle cerebral artery, Hemingway risked landing on the wrong side of the statistical divide. Yet after her ambulance arrived at the Massachusetts General Hospital and she was rushed to the neurology unit for a CAT scan, the images of her brain gave neurologist Lee Schwamm hope. Though the tissue adjacent to the clot was already dead, the surrounding area might still be saved if blood flow could be quickly restored, a process known as reperfusion.
Normally, stroke patients receive high doses of TPA intravenously. But in Hemingway’s case, a chest X-ray suggested that her heart was enlarged and possibly surrounded by fluid, and TPA, coursing through her bloodstream, could cause fatal bleeding. Moreover, her blood clot was so large that it might not respond to conventional intravenous TPA treatment. “Intravenous TPA is good at dissolving small clots in smaller arteries but not so good with clots in major arteries, which can be centimeters long,” says Schwamm, the head of the hospital’s Acute Stroke Service.
Schwamm recommended an experimental procedure intended to deliver TPA directly into the blocked artery, thereby reducing the amount of the drug needed as well as the risk of hemorrhage. The hospital’s interventional neuroradiology team inched a catheter through an artery in Hemingway’s groin, using real-time X-ray images to guide the threadlike wire into the middle cerebral artery. When the catheter tip reached the clot, the endovascular team squirted the TPA. The massive blockage melted away under the direct hit.
Such alternative treatments are few and experimental; they’re used in pilot studies and clinical trials or as a last resort. And they’re available only at large research hospitals and specialized stroke centers. Very few victims benefit, and statistically, improvement has been slow. Between 1991 and 2001, the death rate from stroke fell by 11%—less than half the 25% improvement for deaths from coronary heart disease. Still, progress may finally be picking up, in part through a better understanding of what happens during a stroke and the development of better drugs and combinations of therapies. But another part of the solution involves logistics—quickly getting patients to stroke centers where they can receive state-of-the-art treatment.
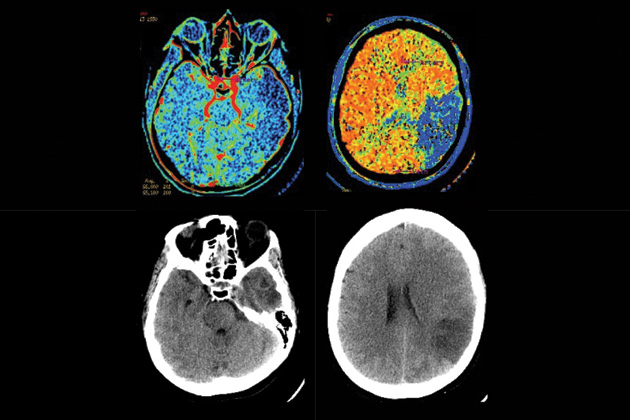
COURTESY THE MASSACHUSETTS GENERAL HOSPITAL
THE FIRST BIG CLINICAL BREAKTHROUGH came in 1996, when the FDA approved TPA for stroke treatment, eight years after the drug was cleared as a heart attack therapy. It’s a genetically modified form of a naturally occurring enzyme whose normal role is to activate plasmin, another enzyme, which degrades the fibrin fibers that form a mesh netting for platelets and other blood cells when a clot forms. By snipping apart the fibrin, plasmin dissolves the clot.
Because the body sometimes needs clots—primarily to stop wounds from bleeding—the bloodstream contains other enzymes that counteract TPA. Normally, the coagulation system maintains a delicate balance, clotting only when necessary, but when that balance is disrupted—for example, when cholesterol builds up in arteries—dangerous clots accumulate. Natural levels of TPA can’t clear them out, but administering extra TPA can dissolve the clots and restore blood flow.
The bloodstream isn’t TPA’s only home. Brain cells also make the enzyme, to promote the brain’s key feature: plasticity, which enables new connections to form among neurons and allows the brain to formulate circuits dedicated to specific tasks—walking, talking or doing the times table. To make those connections, neurons have to send out long extensions, known as axons and dendrites, through a sugary extracellular matrix that cushions and supports brain cells and a network of tiny vessels called the microvasculature. As the brain constantly refines and prunes its neural circuits, TPA helps carve away space in the matrix.
During a stroke, TPA may first have a beneficial effect, dissolving clots. But within three hours, blood vessels in the brain become stressed and prone to leaking. After that, administering TPA intravenously further weakens those vulnerable vessels, allowing blood-borne TPA to seep out and make direct contact with brain tissue. The TPA also activates matrix metalloproteases (MMP), another family of protein-snipping molecules that fray the coating around blood vessels. A vicious cycle ensues as more TPA escapes the vessels to turn on more MMP. Still more TPA reaches the neurons, which it kills directly.
For the stroke treatments to improve, techniques are needed to circumvent or stem this chain of events. Many researchers are looking for combinations of drugs or technologies to extend the usefulness of TPA or other thrombolytics (clot busters), often by protecting neurons or exploiting the body’s own therapeutic responses. For example, Berislav Zlokovic, working at the University of Rochester’s Medical Center, has discovered that activated protein C (APC), a drug widely used to treat sepsis, shields neurons. In experiments described in Nature Medicine late last year, he found that APC turns on an antiapoptosis, or cell survival, pathway. When the two drugs are given together, APC effectively counters TPA’s neurotoxicity in animals. Pending safety and efficacy studies in humans, a cocktail of the two might widen the treatment window for TPA with less collateral damage to the brain, Zlokovic suggests. Both TPA and APC are already FDA-approved drugs and well understood in clinical practice.
A more surprising boost to TPA could come from ultrasound. Several years ago nurses observed that when ultrasound was used to monitor stroke patients while administering TPA, patients did better, says Andrei Alexandrov, then associate professor of neurology at the University of Texas at Houston Medical School and now at Barrow Neurological Institute in Phoenix. Alexandrov suspected that the energy emitted from the ultrasound agitates the clot, allowing TPA to penetrate deeper. To test this hypothesis, he compared 126 patients randomly assigned to receive ultrasound or a placebo following intravenous TPA. The results, published in the New England Journal of Medicine in late 2004, showed 49% of ultrasound patients either having their arteries reopened or achieving dramatic clinical recovery within two hours, compared with 30% of the patients in the control group.
Desmoteplase, a recombinant drug derived from the saliva of vampire bats, is a thrombolytic that could serve as an alternative to TPA. (The bat secretes the compound to keep its victim’s blood from clotting while it feeds.) Now entering final clinical trials, Desmoteplase acts like TPA in chewing up a fibrin clot. But it’s more powerful and more selective, so it doesn’t affect the rest of the coagulation system.
Also nearing the end of the pipeline is NXY-059, or Cerovive—not a thrombolytic but it could potentially be used alone or in combination with TPA. Developed by AstraZeneca, Cerovive is in Phase III clinical trials. Designed to protect neurons until blood flow can be restored, Cerovive is thought to scavenge up free radicals—highly reactive, unstable atoms released after a stroke that can worsen injury to affected areas or trigger programmed cell suicide (apoptosis).
Other efforts at neuron revival involve gases. Oxygen, says Aneesh Singhal, a clinician-scientist in the Massachusetts General Hospital Neurology Department and Neuroprotection Research Lab, has long been controversial. Giving a stroke patient oxygen may relieve oxygen-starved neurons—or increase harmful free radicals. Singhal recently led a small pilot study among patients who arrived too late for TPA. The results, published in Stroke, show improvement in the disability caused by stroke and reduction in the size of MRI lesions (which are usually not reversible) during high-flow oxygen therapy. “We think early oxygen therapy could support stroke-endangered tissue longer and thereby extend the time window for TPA,” says Singhal, who has applied for NIH funding for a larger clinical trial.
Increasing nitric oxide (NO) levels in blood vessel walls might also help. Michael Moskowitz, professor of neurology at Harvard Medical School and a stroke researcher at the Massachusetts General Hospital, explains that NO increases blood flow in the endangered region during a stroke. Moreover, NO promotes the growth of both new blood vessels and neurons, enhancing recovery in the remaining neural tissue, according to Michael Chopp, scientific director of the Neuroscience Institute at the Henry Ford Health Sciences Center. And there are two well-known and widely used drugs that rev up NO and activate other neurorestorative pathways: Viagra and the cholesterol-lowering drugs known as statins. “Both have a similar effect in increasing the production of new blood vessels and new brain cells, which are common denominators for inducing restored brain function,” Chopp explains.
AS ENCOURAGING AS MUCH OF THIS SEEMS, true breakthroughs are likely to come slowly, warns Gregory del Zoppo, associate professor in the department of molecular and experimental medicine at Scripps Research Institute in California. “The brain is still pretty much a black box,” he says. “Every positive outcome has a neural and a vascular component, and at this point, we usually don’t know which is which.”
There’s also a bigger problem. Whatever the advances in stroke treatment, many victims won’t benefit if they don’t find their way to facilities equipped with the best technology and staffed with physicians willing and able to provide the latest therapies. Rather than being taken to the nearest hospital, patients need to be transported to designated stroke centers, where they can be treated effectively. In other words, the emergency network for stroke needs to emulate the system for emergency cardiac units, which in turn followed the one devised for trauma victims.
Hemingway probably owes her nearly complete recovery to such a stroke network in Massachusetts, for which Schwamm was a prime mover. As in several other states, patients are transported to specialized stroke centers that are staffed 24/7 with a neurologist, trained technicians and magnetic resonance imaging or CAT scans in the ER. Helicopters can quickly deliver patients from anywhere in New England. And Schwamm has developed a “telestroke” program, in which a neurologist at a stroke center can consult while viewing the ER scene and brain images on a monitor as doctors treat a stroke patient in a distant hospital. Such logistical initiatives may save more patients than will a pharmacy full of new therapies.
But something else is needed—early recognition. As with most strokes, Hemingway’s came without warning. “One minute I was fine and the next I was numb on my right side,” she recalls. Many might have missed the urgency of the situation, particularly because Hemingway, a young woman, wasn’t a typical stroke patient. And as long as the vast majority of the public—83% at last report—remains oblivious to a stroke’s warning signs, most victims will continue to lose their race against time.
Dossier
“Mechanisms, Challenges, and Opportunities in Stroke, ” by Eng H. Lo, Turgay Dalkara and Michael A. Moskowitz, Nature Reviews: Neuroscience, May 2003. A well-illustrated historical overview of the understanding of stroke biology, brain imaging, treatments and new research directions.
“Exciting, Radical, Suicidal: How Brain Cells Die After Stroke,” by Eng H. Lo, Michael A. Moskowitz and Thomas P. Jacobs, Stroke, Feb. 2005. A helpful summary of recent research about cell death and the neurovascular unit.
“Recommendations for the Establishment of Stroke Systems of Care,” by Lee H. Schwamm et al,Stroke, March 2005. Influential recommendations from the American Stroke Association’s task force for establishing a network of stroke centers, as well as education for prevention, emergency response, treatment and rehabilitation.
Stay on the frontiers of medicine