Published On January 22, 2019
In May 2008, doctors at the Centre for Digestive Diseases in Sydney, Australia, began treating a 73-year-old man for severe constipation. They gave him strong antibiotics and anti-inflammatory drugs, and within weeks, his constipation abated. But something strange—and surprising—also happened: The symptoms of the man’s Parkinson’s disease largely went away. In a report published in The American Journal of Gastroenterology, the doctors called the man’s relief from his neurological disorder “unexpected and dramatic.”
The incident added to a growing body of evidence to support a curious hypothesis—that Parkinson’s disease, unquestionably a disease of the brain, may in some cases begin in the gut. According to this theory, the disease starts with certain proteins misfolding in the gut, which then leads to a chain reaction of aberrant proteins traveling up a nerve to the brainstem. From there, the damage spreads to the rest of the brain.
Although this model of Parkinson’s disease has been kicking around for almost 15 years, recent discoveries have led researchers to consider it more seriously. Epidemiological evidence increasingly points to a link between gastrointestinal (GI) disorders and the risk of Parkinson’s disease, while animal studies are helping researchers understand how, physiologically,the connection between gut and brain might occur. Although the hypothesis remains controversial, some scientists consider the accumulating findings difficult to ignore. “It really divides Parkinson’s researchers into believers and nonbelievers,” says Per Borghammer of Aarhus University in Denmark.
If the believers are right—and most cases of Parkinson’s disease do begin in the gut, in advance of any neurological symptoms—there could be enormous implications. If clinicians had an early start on detecting the disease, they might be better able to slow it, and perhaps prevent the pathology from reaching the brain in the first place.
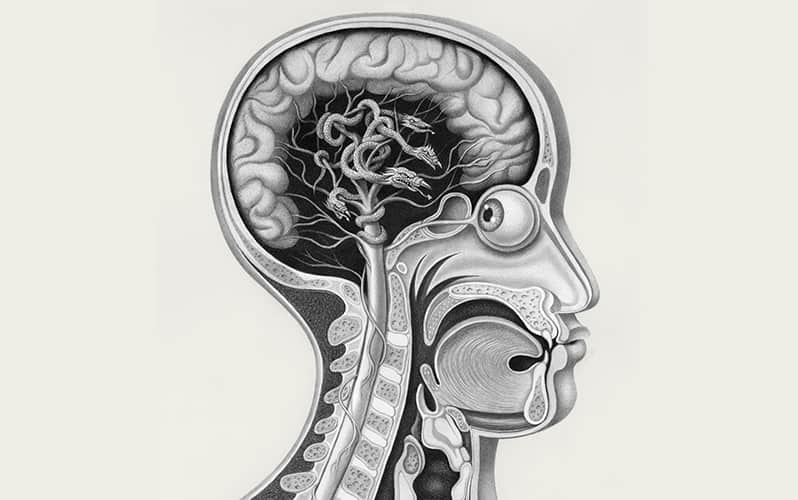
Parkinson’s is the second most common neurodegenerative disease, second only to Alzheimer’s. There is some confusion about how to categorize it—is it a disease, with a singular cause, or a syndrome of similar symptoms that have different pathological underpinnings? Either way, most researchers agree that its main feature is the appearance of motor symptoms that may include tremors, muscle stiffness and slowness and rigidity of movement. A small portion of cases arise from known genetic mutations that run in families. But the form of the disease that has no known cause, the idiopathic version, is much more common, accounting for 80% to 90% of Parkinson’s cases worldwide.
No objective test, such as blood biomarkers or telltale signs in brain scans, can detect the disease, so clinicians diagnose Parkinson’s based on the appearance of the motor symptoms. Autopsies of patients who died of Parkinson’s, however, have shown that the disease likely begins well before those symptoms show up, with the appearance of protein aggregates in the brain called Lewy bodies and Lewy neurites. Named after Friedrich Lewy, a German-born American neurologist who in 1912 discovered these protein deposits in a patient who had died of Parkinson’s disease, Lewy bodies and neurites are primarily made of abnormal forms of a protein called alpha-synuclein. The faulty proteins clump together and are thought to damage neurons in the brain, particularly in a region called the substantia nigra, a tiny cluster of neurons near the top of the brainstem. Especially vulnerable are cells that produce the neurotransmitter dopamine, a lack of which leads to the characteristic motor symptoms.
Those facts about Parkinson’s are largely undisputed. Less certain is where damage to the neurons begins—in the brain or somewhere else. In 2003 a team of neuroanatomists, then at the J.W. Goethe University in Frankfurt, Germany, proposed an entirely novel hypothesis, suggesting that the formation of Lewy bodies and neurites, and hence the harm to neurons, originates in the gut and olfactory bulb. They based this theory on their examination of the brains of 168 people. Of those, 110 had either been diagnosed with Parkinson’s disease or had deposits of Lewy bodies or neurites in various regions of their brains. The other brains served as controls.
Comparing the brains suggested that the disease progressed in stages, with the earliest signs of damage occurring in two areas. The first site was in the olfactory bulb, in the front of the brain, which has led one camp of researchers to wonder if toxins or pathogens in the adjacent nasal cavity might be a factor in Parkinson’s. The other site of damage was in the vagus nerve’s dorsal motor nucleus, a clump of neurons at the bottom of the brainstem. The neurons in this area send and receive signals via the vagus nerve, which extends down into the GI tract and reaches other internal organs.
A chain reaction of aberrant proteins travels up a nerve to the brainstem.
Early damage to neurons in those two brain areas spread into other parts of the brain, eventually reaching the substantia nigra, the cerebral cortex and the neocortex. But the neuroanatomists found that lesions in the olfactory bulb were less likely to extend into other brain areas than were those in the dorsal motor nucleus of the vagus nerve. This prompted them to wonder whether the disorder might be caused by an as yet unidentified pathogen that enters the GI tract, then creeps up the vagus nerve into the brainstem and deeper into the brain.
The first part of their conclusion—that the disease progresses in stages within the brain—was immediately accepted as a major finding, says Borghammer. For years, however, there was much more skepticism about the second notion. That has changed recently. “Those latter ideas have really picked up speed, and they’re talked about more and more,” he says.
The gut hypothesis offers an explanation for one of the more puzzling aspects of Parkinson’s, namely that patients also suffer from constipation and other GI disorders that are governed by involuntary muscles, in addition to the voluntary muscle disorder more characteristic of the disease. The Lewy pathology found in patients’ brains is also found in neurons in the gut and could explain the impaired motility of the GI tract. “Constipation often shows up far earlier than the motor symptoms of Parkinson’s,” says David Sulzer, a neuroscientist who studies the basic neurobiology of Parkinson’s at Columbia University Medical Center in New York.
Further support for the gut-brain hypothesis has come from epidemiological studies. Borghammer’s team, among the first to think of approaching the question in this way, started with the simple notion that if the vagus nerve is a pathway for Parkinson’s disease to go from the gut to the brain, there should be a lower incidence of Parkinson’s disease in people whose vagus nerve had been severed for some reason.
It turned out that many people, decades ago, had undergone just such a surgical procedure—vagotomy—to treat serious peptic ulcers. The vagus nerve stimulates the production of stomach acid, and cutting it was an effective, if drastic, treatment.
In 2015 Borghammer and his colleagues used the Danish National Patient Registry to find all patients who had undergone vagotomy between 1977 and 1996. Analyzing data from more than 11,000 of those patients—as well as from 127,211 people who hadn’t undergone such surgery—they discovered that “if you had a full truncal vagotomy, your risk of Parkinson’s disease was more or less cut in half,” says Borghammer.
A report published in 2017 by a team from the Karolinska Institute in Stockholm used similar data from Swedish medical registers. The researchers identified 9,430 patients who had undergone vagotomy, of which nearly 3,500 had had their vagus nerve fully severed. The dataset was cleaner than the one in the Danish registry, which had muddied the distinction between certain partial vagotomies and full vagotomies. The Swedish researchers matched these patients by age and sex with 377,200 people from the general population. “They found more or less exactly the same thing we had,” says Borghammer: The risk of getting Parkinson’s disease was cut almost in half in people who had undergone a truncal vagotomy. But he points out that even with truncal vagotomy, the risk is not entirely eliminated, suggesting that there may be other pathways for the disease.
Taking a different approach, Inga Peter and her colleagues at the Icahn School of Medicine at Mount Sinai in New York City identified 144,018 U.S. patients who were diagnosed with inflammatory bowel disease (IBD). When those people were matched with 720,090 control patients, the researchers found that patients with IBD were 28% more likely to develop Parkinson’s. “This is indirect but very strong evidence” that disturbances of the gut and Parkinson’s disease are linked, says Peter. “I’m a big believer that that’s the case.” Several other studies have shown similar results, including a September 2018 meta-analysis that found that the overall risk of Parkinson’s in IBD patients was significantly higher (95%) than in control groups.
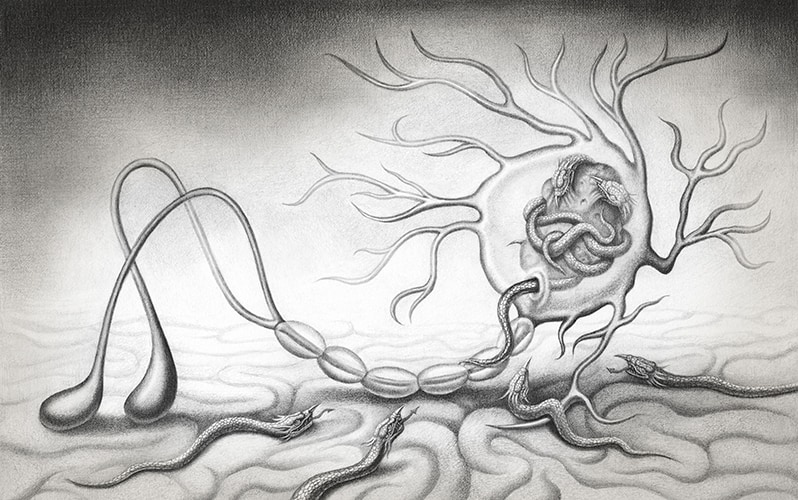
Alpha-synuclein (a) is found in neural cells and in the space between cells. The protein can become misfolded and form short, rodlike fibrils or can clump together to form amorphous protein aggregates (Lewy bodies). Sometimes the cell can clear these, but not always. Neurons have a system to transport important cellular elements along the long, narrow axon, which in the vagus nerve can stretch in a single cell from the base of the brain to the abdomen. Alpha-synuclein aggregates are caught up in this transport system and carried toward the brain. At the terminal, the aggregates can be released by one neuron and taken up by another. If there are too many of these abnormal aggregates inside a neuron, they can lead to that cell’s death. 


To find more evidence of that link, researchers have tried to reproduce parts of this hypothetical process in animal models. Virginia Man-Yee Lee, a neuroscientist at the University of Pennsylvania Perelman School of Medicine in Philadelphia, wanted to know whether Lewy bodies could spread from one region of the nervous system to another. She led a team that injected abnormal forms of the alpha-synuclein protein into different locations in the brains of mice.
In 2012 they reported that just one injection in one spot of the brain was enough to induce a cascade of reactions, resulting in the appearance of Lewy bodies and neurites elsewhere in the brain. That, in turn, led to the loss of dopamine-producing neurons in the substantia nigra and loss of motor coordination in these mice—a pathology similar to Parkinson’s disease.
Their work showed that abnormal alpha-synuclein proteins can induce aberrations in normal forms of the protein, and that those problems can spread. One explanation is that these complex molecules may have the properties of a prion, a type of abnormal protein that acts as a “bad apple,” passing its own malformations on to normal proteins in connecting neurons, which causes a slow-and-steady chain reaction through the nervous system.
If a gut connection were established, the implications for preventing Parkinson’s could be enormous.
To investigate whether such a cascade might travel all the way from the gut to the brain, a team led by Staffan Holmqvist at Lund University in Sweden took brain tissue from a patient with advanced Parkinson’s disease and “homogenized” the tissue in a blender. They prepared a solution containing both normal forms of alpha-synuclein and clumps of misfolded alpha-synuclein and injected the solution into the intestinal walls of adult rats. In examining the animals 12, 48 and 72 hours after the injection, the team detected human alpha-synuclein along the vagus nerve in a pattern that suggested various forms of the protein were being taken up and transported via the vagus nerve to the brain. This was yet another piece of the puzzle: Not only do misfolded alpha-synuclein proteins cause normal proteins to misfold, but the misfolded proteins can be transported inside the long axons of neurons.
When the researchers injected rats with a different protein—bovine serum albumin, from cows—it didn’t progress up the vagus nerve, suggesting that alpha-synuclein is uniquely capable of making that trip.
The hypothesis that Parkinson’s begins in the gut is not without its skeptics. Charles Adler, professor of neurology at the Mayo Clinic College of Medicine in Scottsdale, Ariz., thinks that even if misfolded proteins sometimes do travel along the vagus nerve, it is probably far more common for the disease to begin in the brain, perhaps in the olfactory bulb, and then travel down the vagus nerve. “We believe that it’s a top-down progression,” he says.
The evidence for this alternative theory comes from more than 600 whole-body autopsies done by the Arizona Study of Aging and Neurodegenerative Disorders. Because Parkinson’s develops gradually, if the gut-to-brain hypothesis were correct, you would expect to find Lewy bodies in the gut of some people but not yet in their brains, Adler notes. But he and the other researchers found no cases in which alpha-synuclein pathologies were present in the nervous system in the GI tract but were absent from the brain. While this seems to refute the Parkinson’s-begins-in-the-gut theory, the autopsies are not conclusive, since the progress of the pathology hasn’t been studied in a living person.
If a gut connection were established, however, the implications for detecting and perhaps even preventing Parkinson’s disease could be enormous. For example, a biopsy of the colon in patients who have been diagnosed with IBD might detect protein abnormalities, such as alpha-synuclein aggregates, that could lead to Parkinson’s. That has proven difficult so far, in part because all older patients are likely to have some Lewy bodies and neurites in their colons, even though not everyone will get Parkinson’s. But this research continues as a possible avenue for early diagnosis.
Or perhaps Parkinson’s could be prevented. Inga Peter’s epidemiological study, which found a 28% higher incidence of Parkinson’s in patients with IBD also found that the incidence of Parkinson’s was 78% lower in IBD patients who took anti-inflammatory drugs to treat their IBD.
Their medications, called anti-tumor factor necrosis drugs, aren’t supposed to affect the brain directly because they can’t cross the blood-brain barrier. But the fact that they do seem to have a therapeutic effect could mean one of two things, says Peter. It could be that reducing inflammation in the gut lessens the risk of Parkinson’s disease. It’s also possible that IBD patients, whose gut linings are known to be compromised, may have a leaky blood-brain barrier that lets the anti-inflammatories reach the brain. “We are trying to investigate both of these possibilities,” says Peter.
For Denmark’s Per Borghammer, these are all enticing avenues to explore. Treating the gut to prevent Parkinson’s—perhaps with a drug designed to disrupt the formation of Lewy bodies and neurites in the gut in the first place—would be far simpler than treating Parkinson’s once the pathology enters the brain, Borghammer suggests. “The beautiful thing is that you could take such a drug in very small doses,” because it would only need to be absorbed into the gut wall, he says. That’s in contrast to a drug meant to prevent Lewy bodies in the brain, which would have to be considerably stronger to get across the blood-brain barrier and might be toxic to the rest of the body at that dose.
Some researchers, however, advocate continued caution about a gut-brain connection for Parkinson’s. The basic reason to continue to question this model is that researchers have looked only at animal models and at patients who have died of Parkinson’s, says Sulzer. “We have not yet followed this theoretical progression—from a pathogen in the gut, up the vagus nerve, and then through the neurons in the brain—in a single person to see whether it actually occurs.”
Nonetheless, the community of Parkinson’s researchers is becoming serious about the gut’s role in the disease. “It’s gaining more and more impetus,” says Borghammer.
Dossier
“The Concept of Alpha-Synuclein as a Prion-Like Protein: Ten Years After,” by Jennifer A. Steiner et al., Cell and Tissue Research, July 2018. This paper reviews the current knowledge about prion-like behavior of the aberrant proteins associated with Parkinson’s.
“The Enteric Nervous System in PD: Gateway, Bystander Victim, or Source of Solutions,” by Kathleen Shannon et al., Cell and Tissue Research, July 2018. This article looks at findings that either support or confound the hypothesized gut-brain connection in Parkinson’s.
“Does Parkinson’s Disease Start in the Gut?” by Arthur Lionnet et al., Acta Neuropathologica, January 2018. Researchers use autopsy data to cast doubt on the hypothesis that Parkinson’s disease originates in the gut.
Stay on the frontiers of medicine
Related Stories
- Fertile Ground
The brain, it turns out, can heal itself, and adding stem cells could unleash that toper to treat Parkinson’s, stroke and even depression.
- Bright Future for the Feces Cure
Transplanting healthy human feces became a breakthrough treatment for C. diff infection. Now researchers ask—can it do more?
- Into the Depths
The first endoscope went on display with the help of a talented sideshow performer.