Published On September 22, 2014
ON MAY 23, 2013, The New England Journal of Medicine published a letter from physicians at the University of Michigan and Akron Children’s Hospital in Ohio that may have offered a glimpse into the future. The doctors described how they made an airway splint out of biodegradable polyester with the aid of a 3-D printer. Then they used the flexible splint to repair a collapsed bronchial tube in a baby boy who was struggling to breathe. It saved his life.
The story made headlines around the world, and by including the letter, the Journal seemed to anoint three-dimensional printing as a legitimate force in medicine. The technology employs a variety of manufacturing techniques that build solid objects from the bottom up, layer by layer. Invented 30 years ago, 3-D printing has been adopted as a design tool in the aerospace and automotive industries, among others.
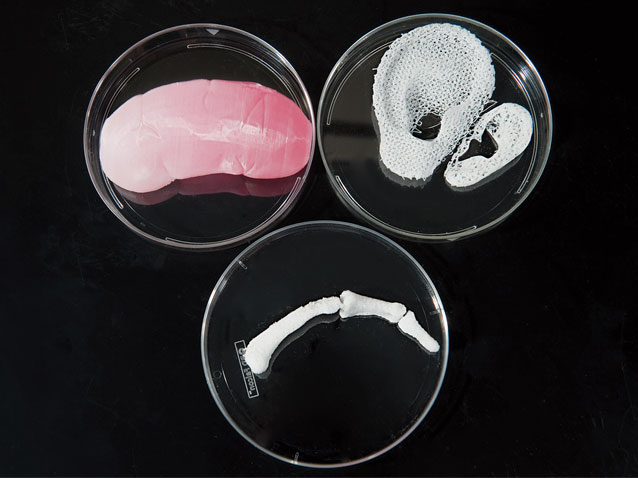
Yet there’s a growing sense that medicine may be the field with the most to gain. While the letter to the Journal seemed to herald the arrival of a new era, the technology has been slowly making inroads in medicine for some time. Surgical supports, such as the splint in Michigan, are one part of the picture, but researchers have also been exploring how to print customized medical devices and even replacement body parts. A new jawbone and several sections of skull have been successfully printed and surgically implanted, including a total skull replacement that Dutch doctors reported earlier this year.
So far, these replacement parts have been made of synthetic substances, often some form of polymer, though it’s also possible to print in other man-made materials, such as metal or ceramic. Actual bioprinting, which uses a very delicate “ink”—human cells—has proven more challenging. But success in that realm could save thousands of lives, and some doctors and bioengineers believe printed organs may one day help ease the enormous backlog of patients waiting for transplants of kidneys, livers and other organs. The replacements might even be constructed with a patient’s own cells, eliminating the need for immunosuppressive drugs to prevent rejection. A major challenge remains: finding a way to keep an organ alive once it’s fresh off the press.
IN 1983, AN ENGINEER NAMED Chuck Hull worked for a company that made equipment to enable durable coatings for furniture and other products, a process that involved aiming ultraviolet light at acrylic-based liquids called photopolymers. Because those liquids turn solid when exposed to light, Hull thought they might help solve a long-standing problem in industrial design. Manufacturers build plastic prototypes of new products to test features during the design phase. However, that has required hiring outside fabricators to mill the models, a costly, time-consuming process.
Hull devised a faster method, called stereolithography (SLA), that uses computer-aided design (CAD) software to create a three-dimensional digital representation of an object on a computer screen. A computer file with that data is fed into an SLA machine, which aims a laser beam into a vat of photopolymer activated by the laser. As the beam moves about according to the CAD file’s instructions, it forms a very thin solid shape. The vat is then lowered slightly and the process is repeated again and again, layer by layer, forming a plastic object. It’s a little like making pottery through the coil method, in which one layer is added to another until the finished shape emerges.
Hull eventually began calling this process 3-D printing, and in 1986 he co-founded 3D Systems, based in Rock Hill, S.C. The company has refined SLA methods and developed other types of 3-D printers, including modified inkjet models that use polymers and other synthetic inks. 3D Systems sells to many kinds of manufacturers, including a growing number in health care, says Lee Dockstader, vice president of business development for the company.
In fact, doctors and makers of medical devices were early adopters of 3-D printing. Surgeons have long used the technology to make models of bone and organs to help them plan operations. In 1992, a team of German scientists reported in the journal Pediatric Radiology that a pediatric cranium model made using SLA was significantly more accurate than one produced with standard milling, though the researchers grumbled that the SLA model took 59 hours to print. Today, producing a child’s skull would take about 10 hours, says Dockstader.
A few months before the University of Michigan team reported its successful implantation of the printed airway splint, there was news of a man who had 75% of his skull replaced with an implant made by Oxford Performance Materials, of South Windsor, Conn. Earlier this year, Dutch surgeons used the technology to replace the entire skull of a woman suffering from a rare bone disorder.
Compared with older manufacturing methods, 3-D printing is faster, cheaper and better at customizing designs, say engineers who have adopted the technology. Many prefer to call it “additive manufacturing” (AM) to reflect 3-D printing’s layer-upon-layer approach. To make skull implants, OPM uses a form of this process called laser sintering. It’s similar to SLA, but instead of using a vat of liquid, the raw material is medical-grade polymer powder.
Other medical device companies have embraced AM. For example, ConforMIS, in Bedford, Mass., uses 3-D printers to make patient-specific surgical guides that doctors use when creating the firm’s custom-fitted knee implants. Meanwhile, 90% of custom-made hearing aids are now produced this way, and every day, a company called Align Technology prints 80,000 to 100,000 models of teeth on which to form their Invisalign clear orthodontic aligners (an alternative to metal braces). And dental lab technicians are making crown and bridge components twice as fast, using a 3D Systems printer the size of a coffeemaker.
Physicians increasingly see a role for 3-D printers in medical clinics too. Massachusetts General Hospital interventional radiologist Rahmi Oklu is working with a scientist at Massachusetts Institute of Technology to find out whether 3-D printers can produce custom-fitting IVC filters, which are devices inserted into the inferior vena cava, a large vein in the abdomen, to prevent blood clots from causing pulmonary embolisms in patients with deep vein thrombosis who can’t be treated with medication. Current filters are one-size-fits-all, but veins come in all widths, and a filter that’s too small can slip and migrate to the heart or lungs, requiring surgery. One that’s too large can poke through a vein’s walls.
Oklu and the MIT scientist have printed a prototype IVC filter, which has the same umbrella-like shape as the mass-produced kind. Oklu hopes to begin testing printed filters in animals soon. He envisions a time when interventional radiology clinics will use 3-D printers to make the filters, as well as aortic stents and other devices, on demand. And that could save money as well as improve patient care, says Oklu’s colleague, Rahul Sheth, a clinical fellow in radiology at MGH, by reducing the need to keep large inventories of devices.
VAULT 49
TISSUE ENGINEERING BEGAN TO emerge as a discipline in medicine in the 1980s. In the traditional form, scientists build a scaffold or a mold out of biodegradable material and then may use a pipette (a chemical dispenser) to seed it with cultured living cells. Then they put the tissue in a bioreactor, providing a warm, moist environment that stimulates cells to grow.
In 1997, Joseph Vacanti, surgeon-in-chief of the MassGeneral Hospital for Children and a tissue-engineering pioneer, published a paper with several colleagues in Annals of the New York Academy of Sciences describing the potential for 3-D printers in tissue engineering. But Vacanti eventually turned his focus to other techniques. “The 3-D printers didn’t have the resolution we required,” he says. One key criterion that early 3-D printers lacked, Vacanti explains, was the ability to produce tiny structures necessary to sustain life, such as blood capillaries that deliver oxygen and nutrients to tissue.
Nonetheless, interest in printing tissue and organs persisted, with some tissue engineers experimenting with inexpensive inkjet printers—they just flushed out ink cartridges and filled them with cells. Modifications to the machines, such as adding platforms that could move up and down, allowed them to print in a third dimension.
One tissue-engineering pioneer who is exploring the potential of 3-D printers to print living tissue is Anthony Atala, director of the Wake Forest Institute for Regenerative Medicine. In 1999, Atala and his colleagues used lab-grown bladders created with traditional bioengineering methods to treat a series of young patients with a birth defect that puts them in danger of kidney failure and caused loss of bladder control. They became the first team to implant an engineered organ in a human. But more than a decade ago, he began experimenting with 3-D printers with the goal of producing human organs. Atala still works in traditional tissue engineering too, but he says 3-D printing has important benefits, such as the capacity to create larger amounts of tissue. “Making things by hand is okay when you’re doing clinical trials,” says Atala. “But if you want to offer this technology to thousands of patients at a time, you need to scale it up.” And 3-D printers may enhance accuracy and predictability. “The technology allows us to lay down cells more precisely, just where we need them,” he says. “We know that when we create one organ, we can create another that’s going to be the same.”
Still, coaxing living cells into forming organs is a deeply complex enterprise, with many challenges that 3-D printing won’t necessarily solve. “It’s not magic,” Atala says. “It’s just a tool.” Now trying to develop a functioning kidney, he believes it may take decades before printable organs are ready for implantation.
The biggest obstacle in building a solid organ—with or without 3-D printing—is supplying its cells with adequate circulation to provide nutrients and oxygen and to eliminate waste. Engineered organs that have been successfully implanted in humans are very thin and hollow, enabling their cells to absorb oxygen and nutrients by diffusion from surrounding tissue. But thicker organs are more difficult. “It takes billions of cells to create a solid organ,” says Rice University bioengineering professor Jordan Miller. “If you try to pack all those cells together in 3-D, the cells at the center quickly die because they can’t get nutrients and oxygen.”
A few years ago, Miller had an idea for solving that problem. He and his colleagues used a 3-D printer to create a network of blood vessels made of sugar, then surrounded it with a gel containing a bath of liver cells from rats. After the gel solidified (to a thickness of about 6 millimeters) around the lattice, the sugar dissolved. “That gave us hollow tubes, which we could pump nutrients and oxygen through,” says Miller. Cells at the center of the bioprinted tissue functioned well for several weeks. Moreover, Miller’s group discovered that capillaries—the tiniest blood vessels—sprouted spontaneously from larger vessels.
Miller says his concept was partly inspired by the work of Harvard University materials scientist Jennifer Lewis, who is using novel techniques and a high-resolution 3-D printer to develop elaborate vascular networks that could one day provide circulation for large, complex bioengineered organs. Lewis’ lab has kept printed tissues healthy for up to a month.
Vacanti believes 3-D printing could be one approach that ultimately solves the problem of building blood vessels in solid organs. And he’s sufficiently intrigued by advances in the technology that he is again considering it, comparing specifications on the latest crop of 3-D printers to see whether any meet his criteria.

WHILE PRINTING IMPLANTABLE organs remains a distant goal, printed flesh could make an impact much sooner, as a tool for developing drugs. Later this year, a San Diego company called Organovo will offer its first product: bioprinted liver tissue. It will be parceled out in small portions, just two square centimeters and a few hundred microns thick. (A sheet of copy paper is about 100 microns thick.) These slivers of liver will be assembled in groups of 24 on the kind of multiwell plates that laboratories use to test experimental drug compounds.
Organovo hopes to convince pharmaceutical companies that its printed liver can help solve a costly problem: drugs that fail or prove toxic when tested in humans or even after they’ve reached the market. Eric David, a physician and Organovo’s chief strategy officer, says the company’s liver product improves on the usual methods for checking toxicity prior to human testing—analyzing a compound in cultured liver cells, laid out in two dimensions on lab plates, and testing on animals. The trouble with these approaches, he says, is that human cells in two dimensions don’t behave like human cells in three dimensions, and animals are poorly predictive of humans.
David says that 2-D testing may be sufficient for finding out whether a drug will kill a liver cell. “But what if you want to ask subtler questions,” he asks, “like what’s the effect going to be over days or weeks?” Will it cause a buildup of bile? Or fat? How does it affect production of albumin and transferrin, critical liver proteins? “You can’t adequately answer those questions for a human being using 2-D cultures,” he says.
Organovo’s long-range goal is to print implantable organs, and it has already created small patches of other organs, including lung, kidney, blood vessels, bone and skeletal muscle that could someday be used to repair diseased or damaged organs. Wholly formed organs are the Holy Grail, says David, “but the industry may get there in bits and pieces.”
Cell biologist Stuart Williams of the University of Louisville’s Cardiovascular Innovation Institute shares that sentiment. Williams heads the institute’s initiative to print the first “bioficial” heart, which he says may be possible within a decade.
To create complex human tissues, Williams and his colleagues have developed a bioprinter/robot hybrid called the BioAssemblyBot, or BAB. Unlike other bioprinters that work only from the bottom up, the nozzles on Williams’ machine can drop back down to add detail. That sort of versatility is necessary for creating the various components of a heart, he says.
Williams’ team has printed blood vessels and stitched them to existing vessels in the hearts of mice and rats, and he hopes to transfer that success to human patients. Within five years he expects to begin using printed vessels to treat patients with coronary microvascular disease, or blockages of the small blood vessels in the heart, as well as people with peripheral arterial disease. Williams is also optimistic that BAB will be able to print tissue for repairing pediatric heart defects too.
If Williams manages to print a human heart, adults with congestive heart failure would be one likely group of recipients. But it will be fine with Williams if he never reaches that goal. “My real hope is we’re going to be treating patients with parts of the heart that we produce with bioprinting long before patients get into congestive heart failure,” says Williams. For example, patients with atrial fibrillation, at risk for congestive heart failure, could benefit from having a new, bioprinted electrical system for the heart. Says Williams, “I hope the technology moves along fast enough that we can say, You know what? We don’t need to build a complete heart.”
Whether it provides replacement organs or merely the components needed to keep them running, 3-D printing appears to have manufactured a role in the future of medicine.
Dossier
VIDEO: Joseph Vacanti of MassGeneral Hospital for Children talks about the future of tissue engineering.
“Bioresorbable Airway Splint Created with a Three-Dimensional Printer,” by David A. Zopf, et al. The New England Journal of Medicine, May 23, 2013. A description of the most high-profile medical application of 3-D printing to date, in which doctors saved the life of a baby boy struggling to breathe with an airway splint made of printed polyester.
“Bioprinting Toward Organ Fabrication: Challenges and Future Trends,” by Ibrahim T. Ozbolat and Yin Yu. IEEE Transactions on Biomedical Engineering, March 2013. Will 3-D printers one day help solve the chronic shortage of organs available for transplantation? This overview discusses the promise of, and the challenges facing, “bioprinting.”
Stay on the frontiers of medicine
Related Stories
- Second Opinion Winter 2015
Readers weight in on the promise of 3D printing in medicine and the importance of telehealth technology.
- New Technology, Old Rules
The use of 3-D printing technology in hospitals and labs has raised new regulatory issues for the FDA.