Published On January 24, 2018
BECAUSE THE HUMAN BRAIN is unique among animals, studies conducted in mice and other laboratory animals often aren’t much help in predicting what will happen in people. Many neurological traits and diseases can’t be accurately studied in mice—for decades the go-to animals for brain research—because the human versions involve cells and circuitry that are quite different in rodents or don’t exist there at all. This problem has bedeviled researchers studying many brain conditions, and it was a particular barrier in research on microcephaly. A baby born with microcephaly has an abnormally small brain, which can contribute to severe neurological defects. But in mice with the condition, brain sizes aren’t reduced in the same way.
In 2012 Madeline Lancaster, a post-doctoral researcher in the laboratory of Jürgen Knoblich at the Institute of Molecular Biotechnology of the Austrian Academy of Sciences in Vienna, was trying another approach. Rather than breeding mice with the rare genetic mutation that can cause microcephaly, she would attempt to grow a tiny version of a human brain with that gene variant. If she succeeded, this so-called organoid—“a brain in a dish”—could give researchers a new window into how the condition unfolds in a developing human fetus.
An organoid is a simplified version of an organ grown from a single human cell. Organoids are small, typically about the size of the head of a pin, though some may grow slightly larger. For her brain organoids, Lancaster started with skin cells from a patient with microcephaly, which carried the gene known to cause the condition, and also from a healthy control. She “reprogrammed” these mature skin cells to become induced pluripotent stem cells (iPS), which can become any type of cell in the body. Then she coaxed these iPS cells to develop into tiny versions of her subjects’ brains.
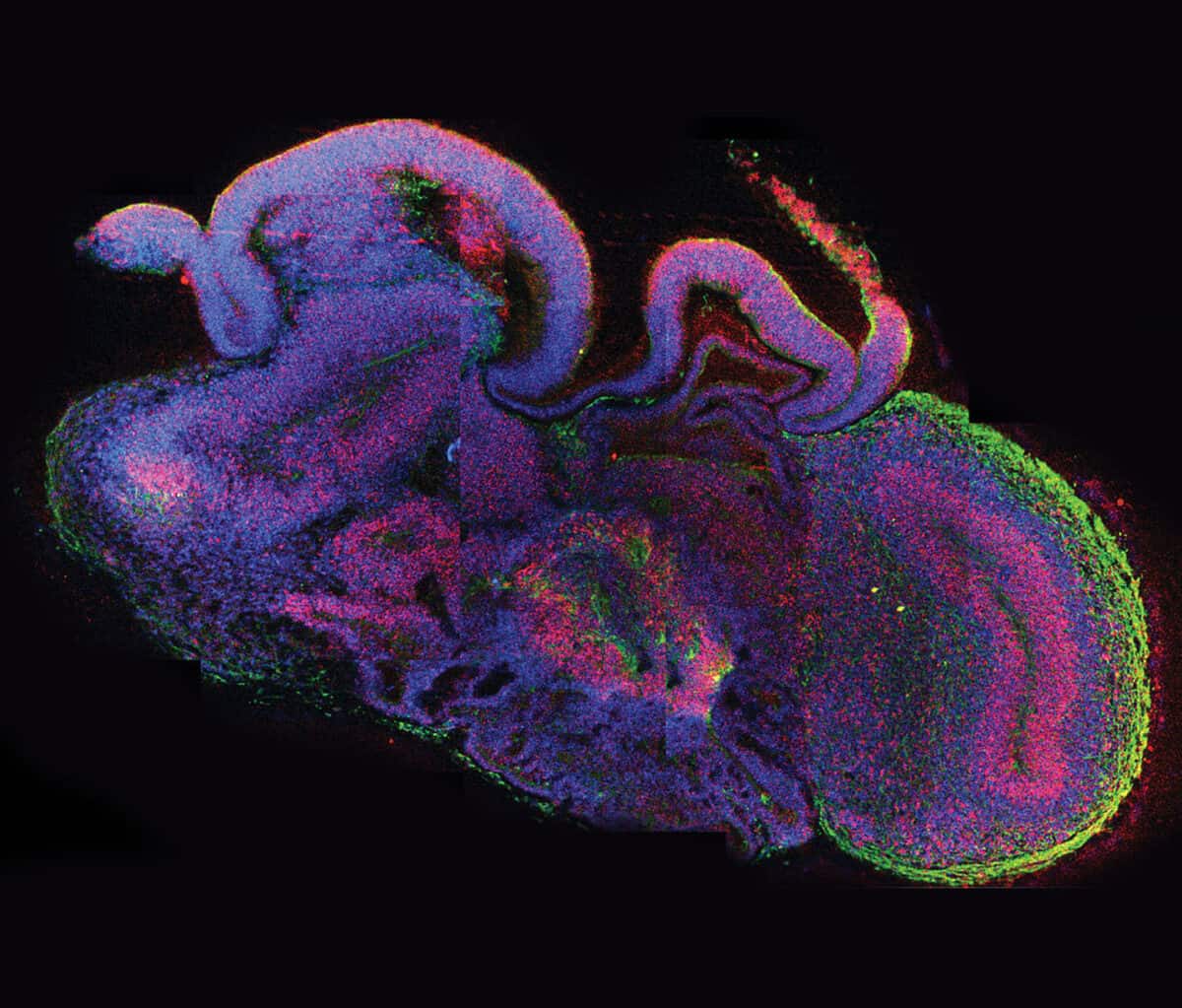
During the next two to three months, the cells began to organize themselves into layers and clusters of different cell types, paralleling what happens during the first eight to 10 weeks of human fetal brain development. Some cells differentiated themselves into various types of the nerve cells (neurons) found in several brain regions, while others remained “neural progenitors,” creating a reservoir of potential neurons to be used later. Producing such self-assembling organoids “doesn’t require any super-sophisticated bioengineering,” Knoblich told Nature in 2015. “We just let the cells do what they want to do, and they make a brain.”
In the organoids that Lancaster had derived from a healthy person, the growth of the hindbrain slowed as the forebrain grew—reflecting what happens as a normal human fetal brain develops. Organoids grown from the cells of a patient carrying the gene for severe microcephaly, however, didn’t grow as large because those brain regions didn’t develop properly. Further research showed that too many neural progenitors in these organoids had become neurons early on, leaving the developing brain without the resources it would have used to enlarge the forebrain.
Lancaster and Knoblich published their work in Nature in 2013, at a time when microcephaly was still rare. But two years later the Zika virus outbreak hit, and physicians noticed that many women bitten and infected by virus-carrying mosquitoes gave birth to babies with microcephaly. To determine whether the Zika virus caused this, a number of independent teams of researchers—including two in Brazil and one at the University of California, San Diego—created brain organoids from healthy human cells and infected some of them with the Zika virus.
Most of the Zika-infected organoids grew to barely half the size of their uninfected counterparts. The Zika virus also replicated the genetic defect in another way, depleting the progenitor cells and causing similar development problems to the ones that Lancaster had observed in Vienna. This experimental proof that the Zika virus caused microcephaly came quite rapidly, and other teams working with Zika have continued to use organoids to test therapeutic interventions and to probe why only some strains of the virus appear to result in the condition.

Since the first organoids were created less than a decade ago, their uncanny ability to mimic in miniature the development of real organs in the human body has caused a quiet revolution in many areas of medical research. Some of their most dramatic contributions, however, have come in studies of the human brain. “Organoids provide a great opportunity to study certain brain disorders that are overtly human, affecting the highest brain abilities that we have as human beings,” says Paola Arlotta, a professor of stem cell and regenerative biology at Harvard University who studies the mammalian cerebral cortex.
Organoids may also break new ground in the pursuit of personalized medicine—treatments targeted to one person’s genetic characteristics. Although organoids can also be created from embryonic stem cells, an organoid derived from a patient’s own cells carries the complete genome of that person. While such a cluster of personalized cells is not a perfect replica of the organ that exists in that person’s body, it can be experimented on and used to gauge the effectiveness of potential therapies, providing a powerful new tool to help researchers understand how diseases develop and how to treat them.
HANS CLEVERS AT THE Hubrecht Institute in Utrecht, the Netherlands, developed the first organoids from mice in 2009, and the first human organoids in 2011. At the heart of Clevers’ work were adult stem cells, a type of cell that can replenish itself while also maintaining the ability to change into the many types of mature cells that a particular tissue or organ requires. For his experiments, Clevers used human intestinal stem cells that his lab had discovered in 2007.
Researchers had developed the technologies needed to create organoids years before—how to grow cells in culture, how to isolate stem cells from human tissue, and how to coax the stem cells, undifferentiated and immature, to become specific types of cells at later stages of development. But when such cells were grown in a laboratory dish, they adhered to the flat surface of the liquid medium they were grown on, spreading out in a thin layer. Confined to two dimensions, the cells couldn’t form the integrated structures of developing tissue.
Previous research had shown that if cells are grown in a medium called Matrigel—firm enough to support cells above a dish’s flat surface and pliable enough for cells to reshape as they grow and multiply—cells are able to develop in three dimensions as they would in the body, assuming the various shapes, layers, compartments and relationships within a particular organ. Clevers added his intestinal stem cells to this gel, and also introduced chemical growth signals that encouraged the stem cells to begin maturing. The intestinal organoids that grew from these elements developed several of the singular characteristics of that tissue type, including small knobby protrusions (crypts) that in full-size intestines serve as receptacles for stem cells.
Meanwhile, others in Clevers’ lab were using similar techniques to grow organoids of the stomach, pancreas, prostate, breast and liver. Researchers elsewhere quickly adapted the methodology to create additional organoids—of the eye, lung, heart and brain—and their associated diseases, including cancers. Researchers now have modeled the majority of human organs, and organoids are being used to study everything from normal development and basic biology to a plethora of disorders.

WHAT SETS ORGANOIDS APART as a research tool is their inherent humanness. Conducting experiments on tissue that is very similar to the kind found in the body helps researchers in many specialties, but nowhere so much as in studying brain diseases, especially those for which treatments have long eluded researchers. “We’ve learned a lot about the brain from mice, but I think we can all agree that mice and humans are very different,” says Li-Huei Tsai, a neuroscientist at the Picower Institute for Memory and Learning at MIT who studies the neurobiology of Alzheimer’s disease. A number of promising drugs for Alzheimer’s have, for instance, worked in mice, but when they reach clinical trials with humans “an astonishing 99% of them fail, and it has been 15 years since the last treatment, memantine, was approved,” she says.
There’s also a wide gulf between what seems to work in mice and what actually helps people who have neuropsychiatric disorders, says Steven Hyman, director of the Stanley Center for Psychiatric Research at the Broad Institute of MIT and Harvard. “Our most effective treatments in psychiatric disease were discovered serendipitously more than a half century ago,” Hyman says. “And despite decades of research in mice, we still don’t know how they really work.”
Not only can organoids potentially offer a better model for human disease, they can also be surprisingly easy to coax into being. Given the correct chemical prompts, cells follow their internal instructions and spontaneously organize themselves. “We kept them healthy, and without giving them many instructions on what kind of cells they should become they produced many of the cells present in the human brain and achieved the formation of complex tissue,” says Arlotta, describing the brain organoids she used in research published in Nature in May 2017.
For that study, Arlotta was looking at a stage of brain development later than the one studied in the microcephaly and Zika experiments, which modeled only what happens early on during pregnancy. So she modified the Lancaster system to allow her organoids to survive and develop in culture for longer than anyone else—more than nine months.
During gestation, a human brain generates the kinds of cells and circuits needed to carry information. Neurons, the main communicators, form connections with other neurons via structures called dendrites, which receive the incoming electrical signals used to communicate information within and beyond the brain, and axons that pass along the message to the next cell across synapses, the gaps between cells. “These long periods of development in the dish allow many types of cells to form and mature. Importantly, neurons acquire properties of mature cells, most notably dendritic spines, the structures that form on dendrites and receive synapses,” she says.
Arlotta is now using this system to study organoids derived from patients with autism spectrum disorder and other psychiatric illnesses as she searches for their underlying mechanisms.
Yet while autism begins during brain development, and it makes sense that a developing organoid could serve as a model, looking at diseases that affect people toward the end of their lives would seem more difficult. Many scientists, concerned about the finite lifespan of organoids, questioned whether they would be useful for research on conditions of aging.
For a 2014 study, Rudolph Tanzi, director of the Genetics and Aging Research Unit at Massachusetts General Hospital, Doo Yeon Kim, assistant in neuroscience at MGH, and their team were able to create an organoid that they called “Alzheimer’s in a dish.” In this three-dimensional culture, grown from human stem cells converted into neurons harboring genetic mutations known to cause the inherited, early-onset form of the disease, the researchers observed some of the characteristic features begin to develop. Plaques made of the protein amyloid were on the outside of cells, and they triggered tangles of a second protein, tau, within neurons—just as they were in the dissected brains of people who had Alzheimer’s. This work was published in Nature in November 2014 and earned the researchers a Smithsonian Ingenuity Award in 2015.
Building on that work, for a 2016 study in PLOS One, Tsai’s lab used cells from three patients with an inherited form of Alzheimer’s as well as cells from two healthy people to create brain organoids. Within two months, those derived from the Alzheimer’s patients began secreting high levels of amyloid protein, which clumped together in the spaces between neurons, resembling the formation of plaques in a fully formed brain. Within three months, the neurons in the organoids also had formed tau aggregates, further bolstering the hypothesis that amyloid plaques precede tau as Alzheimer’s disease develops.
ONCE TSAI’S LAB HAD discovered that organoids could acquire Alzheimer’s-like traits, they moved on to another puzzle: Could those characteristics be prevented or treated? Researchers exposed another set of organoids derived from the same Alzheimer’s patients to experimental compounds known to prevent the secretion or accumulation of amyloid, and they discovered that the plaques didn’t form. (The compounds have not yet been developed for therapeutic use in people.)
That work underscores a chief benefit of all organoids—that they can be used to try out potential therapies, with results that are likely to be similar to what would happen in people when a treatment advances to human clinical trials. Moreover, because organoids can be grown quickly and in huge quantities, researchers can interrogate them repeatedly and mercilessly. How does a particular treatment affect human tissues? Is it toxic? If a suspected disease gene is inserted into the starter cells, what happens to the organoids’ development and function? If organoids are cultivated from people who share a diagnosis for a complex disease—diabetes, Alzheimer’s, schizophrenia, heart failure, celiac disease—will there be differences in how the cells from different people develop, and how the organoids respond to the same drug?
Because organoids are easy and relatively inexpensive to grow and can be created from a particular person’s cells, they might also be extremely useful in personalized medicine, helping tailor a treatment the way some cancer treatments can be targeted to the genetic makeup of a tumor. As organoid techniques improve, researchers will be able to screen a compound on the very patient they are trying to treat, and test the efficacy of new and existing drugs on a patient’s own cells. “It will be revolutionary,” says Arlotta.

IN DRUG DEVELOPMENT, HOWEVER, the remarkable complexity of organoids can sometimes be a distraction. As their cells begin to organize into larger structures, organoids may begin to diverge in important ways from each other, becoming individualized much as a particular person’s organs do. With diversity comes unpredictability, and that’s a problem for testing new treatments, a process in which standardization and reproducibility are essential.
One way around that problem is to work with another kind of 3D cell culture known as a spheroid, which hasn’t yet gone too far down the path of development and still contains mostly identical, similarly differentiated cells.
Much of the work geared toward drug discovery and testing uses spheroids. Lee Rubin, professor of stem cell and regenerative biology at Harvard University, employs them to study spinal muscular atrophy (SMA), a neurodegenerative disease affecting children that is similar to amyotrophic lateral sclerosis (ALS) in adults. “Because I’m interested in developing therapeutics, I need a system that generates billions of human neurons that have the particular mutations for this disease,” he says. Those neurons need to be identical to each other, and to achieve that consistency, Rubin and his colleagues are willing to sacrifice some of the structural complexity they could get with more fully formed organoids. He can control what the spheroids’ cells become—muscle neurons or glial cells, or even muscle or gut cells—by adding particular chemical cues to the medium in which they grow. But because the spheroids were created from a cell carrying the SMA gene, all of the cells, of whatever type, also have that mutation.
Rubin first used spheroids to confirm that, as expected, motor neurons die if they have the disease mutation. “But we also discovered something surprising,” he says. Researchers had assumed that the SMA gene, essential for the survival of motor neurons, affected only that particular type of neuron. But the SMA mutation also generated defects in the other tissue cells—for muscles, gut, lung and thyroid—he derived from the iPS cells they used. That suggested that children with SMA might also suffer other problems affecting muscle tone or digestion, for example, that hadn’t been known or discussed.
To find out whether this lab finding applied to children with SMA, Rubin collaborated with Isaac Kohane, who chairs the department of biomedical informatics at Harvard Medical School, to search some 60 million electronic medical records. They identified about 500 children with SMA and found that they did indeed have the kinds of problems the stem cell work had predicted. No one had connected those symptoms with the underlying genetic condition because those other problems didn’t involve motor neurons.
“By studying cells in vitro we discovered new information and pathologies that were true in actual patients,” Rubin says. “This back and forth between human and laboratory studies is leading to new ways of thinking about treating these kids.” For example, knowing that the SMA mutation causes debilitating muscle weakness, researchers might be able to target treatments to address that problem even if they don’t have a therapy that would affect the motor neurons themselves.
Rubin and Kohane believe that using models derived from human stem cells in conjunction with electronic medical records could provide a wealth of information about the subtypes of complex diseases, perhaps leading to individualized treatments.
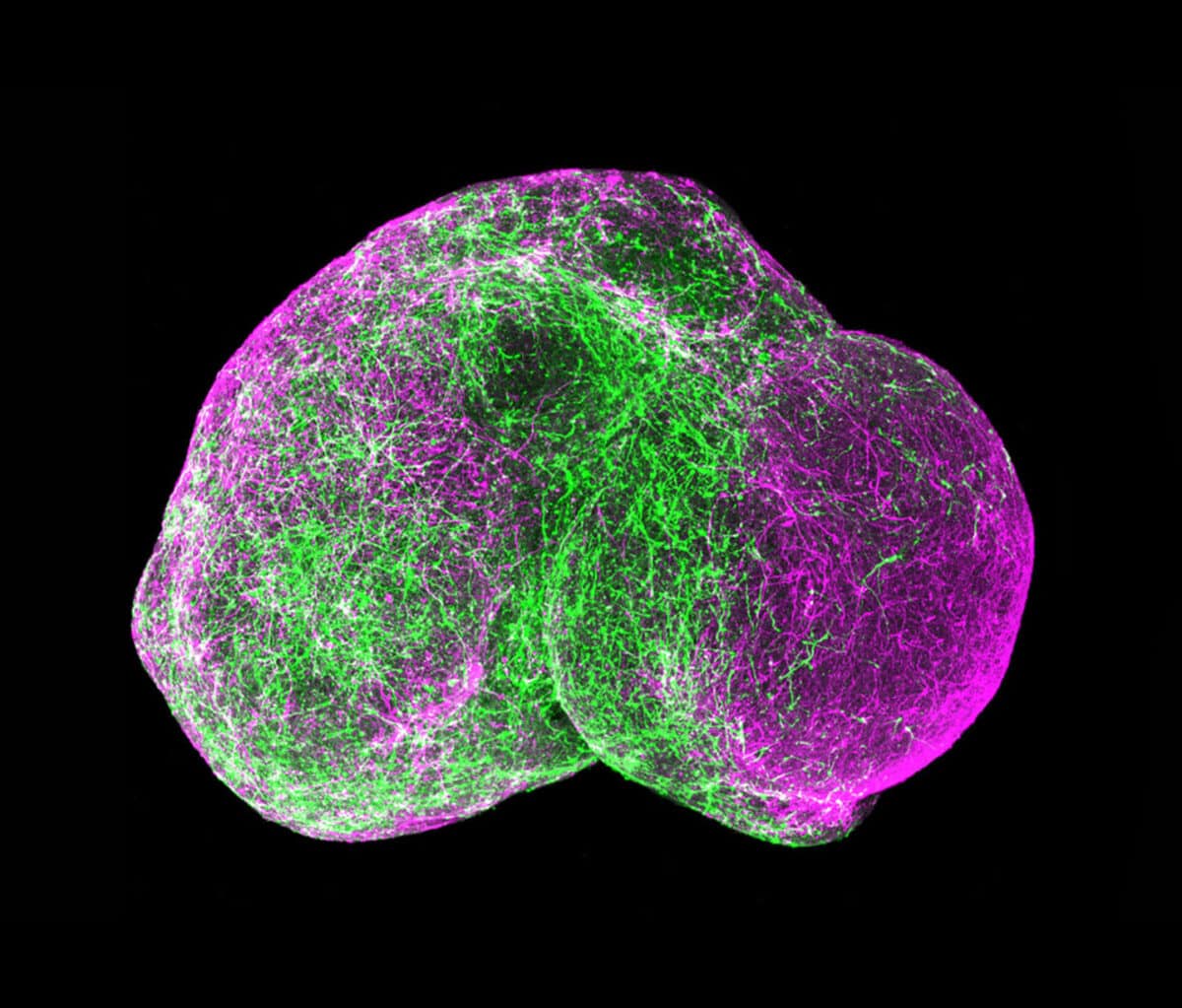
The Rise of the Organoid Learning how to grow these tiny, rudimentary organs required a long series of discoveries, with contributions by a global contingent of research teams. 1951A lab at Johns Hopkins Hospital in Baltimore keeps a strain of human cancer cells alive and propagating indefinitely outside the body. Repeating this feat with normal cells proves more difficult. 1998Researchers at the University of Wisconsin isolate human embryonic stem cells, which renew indefinitely outside the body and can become the precursors to different tissue types. 2003Adult stem cells are isolated from the human mammary gland. These can also self-renew and differentiate into breast cell lineages. They are maintained in a 3D culture using Matrigel. 2007A team at Kyoto University in Japan show how to make embryonic-like cells from human skin cells, obviating the need to use human embryos and reinvigorating human cell culture research. 2011Hans Clevers at the Hubrecht Institute in the Netherlands grows human intestinal organoids using stem cells. They mirror key structural and functional features and can be used to model several disorders. 2012At the Austrian Academy of Sciences in Vienna, a group produces the first brain organoids. They use the tissue to study microcephaly, a disease that in humans causes abnormally small heads and brains. 2015Following the Zika virus outbreak in South America, several teams of researchers use the Austrian method of creating brain organoids to determine if and how the virus causes microcephaly. 2016Neuroscientists use organoids to model later stages of brain development, and show that multiple brain organoids can be fused to replicate more complex functions.
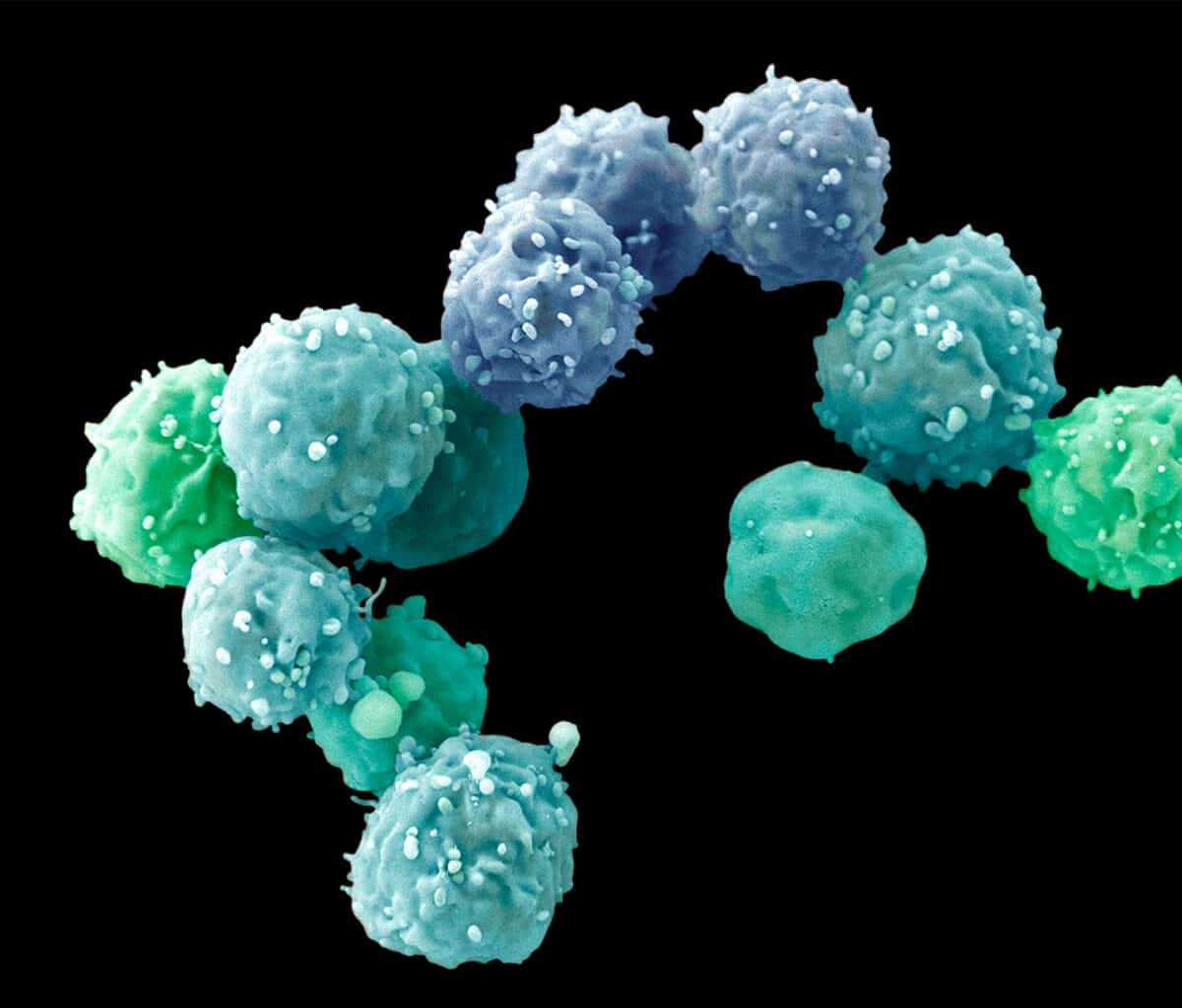

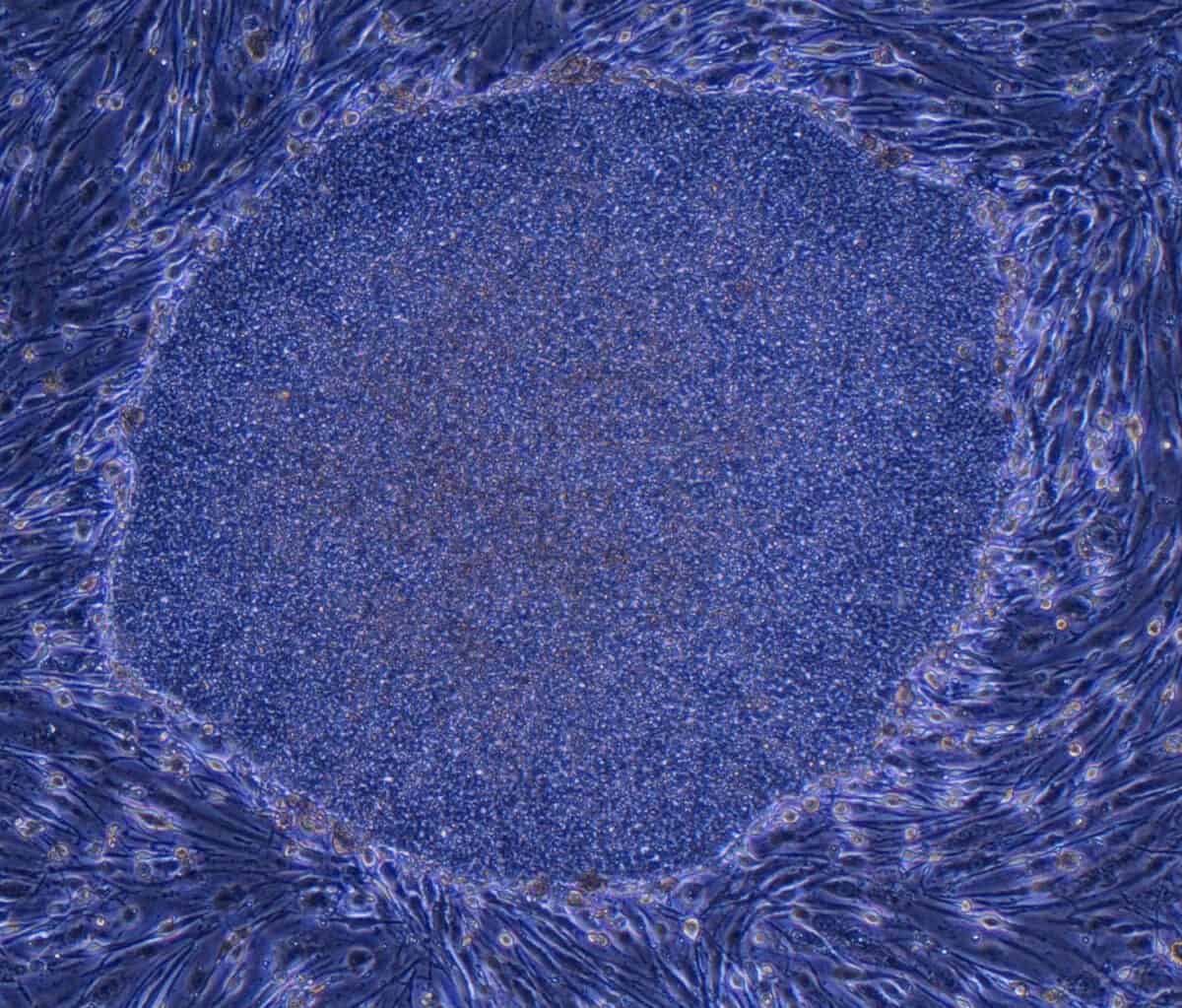
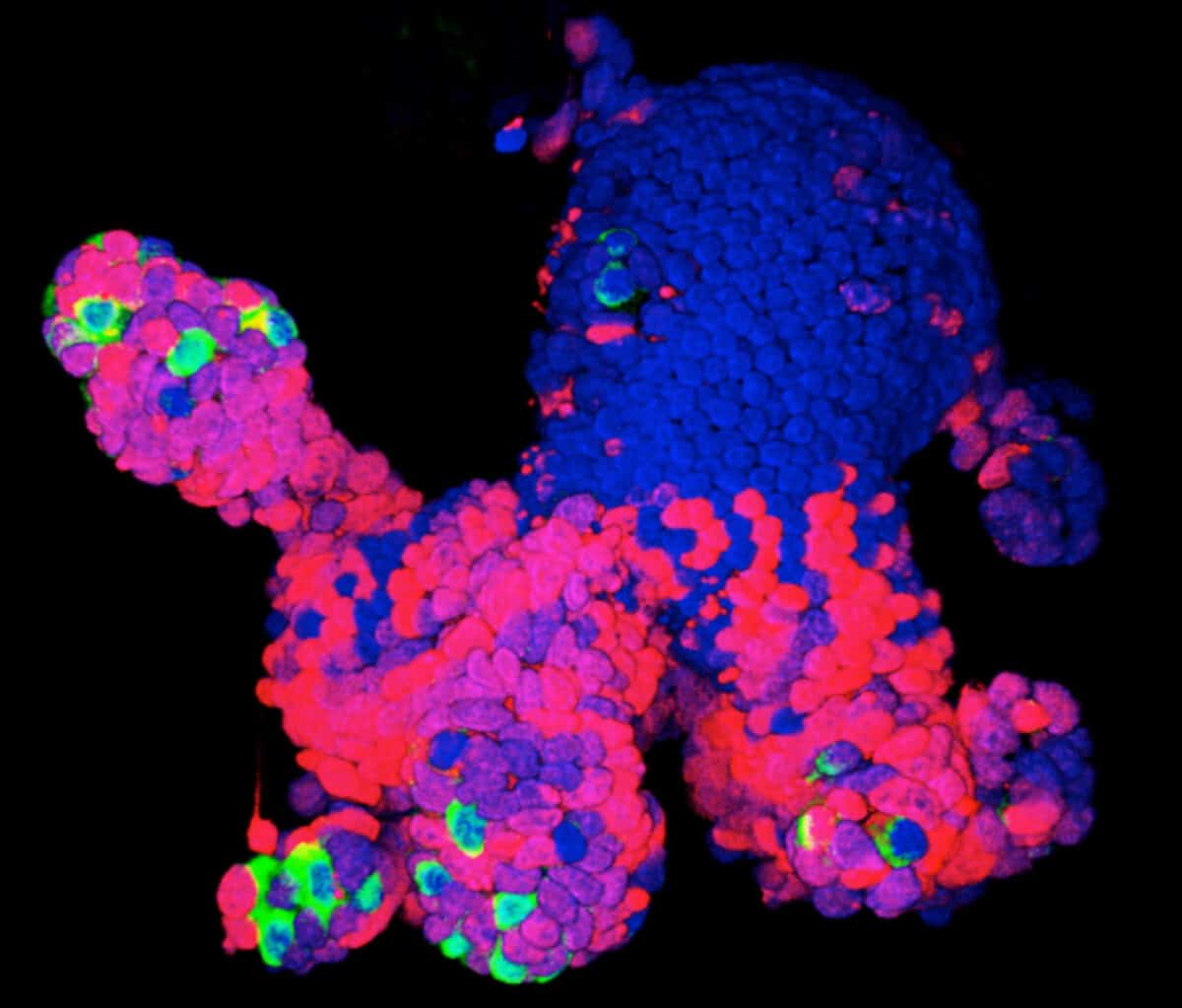
AS POWERFUL AS BOTH spheroids and organoids have become, they can’t accomplish everything scientists might wish for. “We’re excited but cautious,” says Guoping Feng, a neuroscientist at MIT who studies neuropsychiatric disorders. For example, brain organoids so far can feature only relatively young neurons, and some genes that scientists would like to study don’t become active until much later stages of brain development. Diseases such as adult-onset diabetes, kidney failure, heart disease and cirrhosis of the liver afflict fully mature organs and so may be hard to model. (That’s not always the case, as the organoid work on Alzheimer’s suggests.)
More generally, organoids aren’t completely faithful to what happens in a human body, in which, figuratively speaking, no organ is an island. The heart depends on lungs for oxygen, and lungs depend on the beating heart for blood. “Also in real life, organs have an epithelial lining, a vascular component, connective tissues and immune cells, none of which can yet be replicated in their normal physical arrangement in organoids,” says Donald Ingber, founding director of the Wyss Institute for Biologically Inspired Engineering at Harvard University. Most diseases also have an inflammatory component in which the body’s misdirected immune responses contribute to the sickness, and organoids don’t have that either.
Ingber prefers “organs-on-chips,” another modeling approach that lines microchips with human cells—or, more recently, human organoids. He can connect a liver-on-a-chip to a gut-on-a-chip, for example, via blood vessel channels to give a better approximation of the interactions that occur in the body. Still, organoids are easier to engineer and less expensive than organs-on-chips. This may make organoids more suitable for rapid, high-volume screening of potential treatments in drug development and the practice of personalized medicine. Even then, Ingber notes, “you need to know how the drug crosses in and out of the bloodstream across the blood vessel wall, and from one tissue and into another tissue”—something organoids can’t yet test.
That ability may come in the next frontier of organoid development, as researchers look to culture various types of stem cells together to create organoids that incorporate additional features. For example, adding endothelial cells of blood vessels to the initial cells of a culture might enable organoids to spontaneously create a working vascular system. Researchers are also beginning to “fuse” different organoid types to approximate the kinds of linked systems Ingber achieves with organs-on-chips.
Not so long ago, the advent of powerful genomic tools and genetic engineering techniques made it seem that studies involving mice engineered to carry human disease genes would be the best approach for exploring human disorders, superior to looking at cells isolated in a laboratory. Now, however, the ability to grow these three-dimensional cellular models may begin to challenge the supremacy of mouse models in research. Organoids allow researchers to conduct revealing human studies in an out-of-body sort of way.
“We need a range of models to learn about the connections among genes, molecules, cells, synapses and circuits in normal and abnormal cognition and behavior, and to help us understand the risks of particular patients,” says the Broad Institute’s Steven Hyman. Although he is talking about neurological disorders, the message applies broadly across the field of medicine. “Ultimately, we have to get a human model for human diseases so that we can expand human experimental biology in an ethical way and ensure that better, safer drugs get to patients faster,” he says. Tiny organoids in the lab are bringing researchers closer to that model.
Dossier
“Zika Virus Depletes Neural Progenitors in Human Cerebral Organoids Through Activation of the Innate Immune Receptor TLR3,” by Jason Dang et al., Cell Stem Cell, August 2016. This study uses recently developed techniques for growing brain organoids to discover how the Zika virus causes microcephaly in infants.
“The Boom in Mini Stomachs, Brains, Breasts, Kidneys and More,” by Cassandra Willyard, Nature, July 2015. This article provides an overview of the wide-ranging applications of organoids.
“Modeling Development and Disease with Organoids,” by Hans Clevers, Cell, June 2016. This article outlines how organoids can be used to model various human pathologies “in a dish” and how patient-derived organoids may be able to predict drug response in a personalized manner.
Stay on the frontiers of medicine
Related Stories
- Organs in Miniature
Microscopic models—half living, half not—may prove more reliable than animals in explaining human disease and testing therapies.
- Why Plaque Attacks
A bold theory—that the brain-tangling proteins of Alzheimer’s disease may form to fight infection—could spur new research.
- Making the Leap
Ten years ago, researchers coaxed normal adult cells into stem cells for the first time