Published On January 15, 2008
IN 1965, WHEN LIPIDOLOGIST WILLIAM CASTELLI was recruited to run the laboratories of the Framingham Heart Study, then in its seventeenth year, a colleague advised, “I’d steer clear of Framingham. Every fifth guy in that town has a heart attack by age 60.” The warning was of no small concern. Everyone in Castelli’s immediate family had heart disease or had died from it, and his own cholesterol level would reach 260 mg/dL (milligrams per deciliter), exceeding that of many study participants who were succumbing to heart attacks.
But Framingham, Mass., was no anomalous industrial town killing off residents with mysterious toxins. On the contrary, Framingham epitomized Anytown, U.S.A.; the nation was facing an epidemic of heart disease. “Physicians then considered average to be normal, but the last person in the world you’d ever want to emulate was the average American,” Castelli says. “Doctors thought nothing of telling patients that any total cholesterol level below 300 mg/dL was normal. That was devastating advice; 90% of Framingham study participants whose total cholesterol even approached 300 had heart attacks.”
Extrapolating from such observations, the Framingham Heart Study (FHS) has played the role of America’s medical nag for 60 years now, challenging the lifestyles that contribute to chronic diseases as well as physicians’ misperceptions about diagnosing and treating those diseases. Arguably the world’s longest-running and best-known epidemiological study, Framingham can claim such landmark discoveries as linking cigarette smoking to heart disease; dispelling the myth of benign hypertension; showing that hormone-replacement therapy increases rather than decreases the risk of heart disease (long before the Women’s Health Initiative came to the same conclusion); and finding that exercise reduces heart disease instead of causing it.
All part of conventional medical wisdom today, such findings were almost always greeted with skepticism, if not outright scorn. “Every time we asserted something, it was said to be bunk,” says William Kannel, who joined FHS in 1951, became its second director and, at age 84, still does research for it.
Now, though, after publishing 2,000-plus peer-reviewed papers based on the examinations of study participants (there have been almost 15,000), the Framingham Heart Study is recognized as a singular modern medical achievement—and is firmly focused on what could become its twenty-first-century legacy. As the study’s participants have aged and developed myriad diseases, and as new diagnostic tools have entered the scene, Framingham researchers have gathered ever more data, broadening the study to include observations on such disorders as Alzheimer’s disease, osteoporosis, arthritis and cancer.
“While the ‘classic’ Framingham study was all about finding traditional risk factors related to disease, the modern Framingham is about discovering the genetic variations and other biomarkers that underlie blood pressure, lipids, obesity, coronary heart disease, stroke, blood disorders, bone density, dementia—and I can keep going,” says Christopher O’Donnell, Framingham’s associate director and the scientific director of its genomewide association study. Although there are dozens of research studies around the world looking for disease-causing genes, only a few are large-scale population studies. Having the DNA of thousands of people spanning three generations puts Framingham in a unique position to contribute to the next biomedical frontier—uncovering the molecular basis of disease.
IN 1948 LIFE WAS GOOD, or so it seemed. The postwar economic boom had transformed America into a land of plenty, with cars, televisions, cigarettes and second helpings of meat and eggs for just about anyone who wanted them. But prosperity came at a price. People were dropping dead of heart attacks, and physicians in the 1940s had limited understanding of the cardiovascular system, let alone of treatments for heart disease. A quarter of heart attacks were recorded on death certificates as “acute indigestion” or “cause unknown,” according to Daniel Levy, FHS’s current director and author of A Change of Heart: Unraveling the Mysteries of Cardiovascular Disease.
Yet, if general practitioners of the day weren’t willing (or able) to recognize that at least half their patients were dying from heart attacks and stroke, the U.S. Public Health Service was sounding the alarm. In 1948 it sponsored a 20-year epidemiological study that would follow a group of healthy people in one community and observe changes in their health, including the development of heart disease. The government agency provided initial funding of $500,000.
Such a study of a chronic disease had never been attempted in the United States, though a few years earlier, cardiologist Paul Dudley White at the Massachusetts General Hospital had begun following men who’d already had heart attacks. White had hoped to find the causes by backtracking through medical histories and information about the men’s lifestyles. But spotty memories confounded him. “He concluded that the study would have to be done the other way around—to start with healthy people and wait for them to develop heart disease,” Levy says.
So Framingham would look forward, not back, recruiting 5,209 healthy subjects—about half the town’s population between ages 30 and 60—and systematically recording data on their diet, physical activity, smoking, family history and medications. Each participant also underwent an extensive physical examination every two years. Women were included in the study as a kind of control group. Because they were mistakenly thought to have some kind of innate protection against heart attacks, women were expected to teach useful lessons that could be applied to men.
When the town of Framingham’s physicians learned that their patients were to be recruited for a federally funded project, they complained that they didn’t want the government peering into their practices, and they worried they would lose their patients to the study’s doctors. So the first director, Thomas Royal “Roy” Dawber, promised that Framingham would never treat participants but rather would notify physicians of any findings that required their attention. And the town’s physicians were invited to be the first to receive a complete FHS exam, so they could answer patients’ questions about the study.
If Dawber was successful in convincing community physicians of the importance of a heart study, he and the other Framingham researchers had a much bigger challenge in getting them to believe the data. Then-prevailing views of heart disease held that it was either a natural part of aging or a specific biological phenomenon with a single cause—in the way that tuberculosis, for example, is directly tied to the tubercle bacillus. But as study data trickled in, Framingham researchers began to hypothesize that chronic diseases such as stroke and diabetes result instead from several interrelated causes—“factors of risk,” as Dawber and Kannel termed them, coining a variation of the phrase now at the heart of any conversation about disease and how to prevent it.
“Framingham showed you can take measurements done in any physician’s office, such as tests of cholesterol, and predict the probability that someone will have a heart attack or a stroke within 10 years,” says Philip A. Wolf, who joined FHS in 1967 as a stroke researcher and is now its principal investigator. “But the study also demonstrated that if people make particular changes in their lifestyles, they can substantially reduce their risk of disease.” It was a revolutionary idea—and the dawn of preventive medicine.
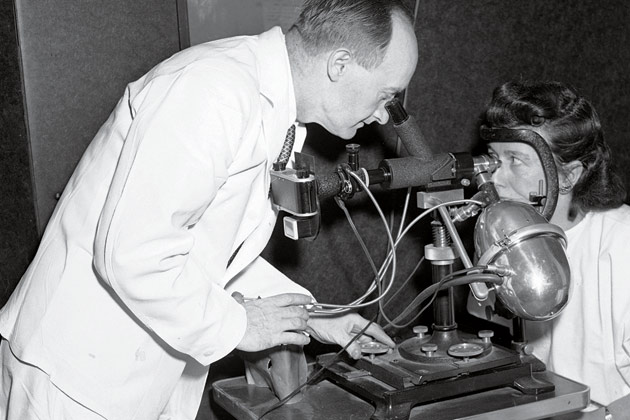
IN 1948, WHEN EVELYN AND AURELE DESROSIERS joined the study at the urging of a neighbor, they knew only that they were being promised an extraordinary level of medical care. And indeed, they soon found themselves undergoing what struck them as astonishingly thorough examinations, with blood tests, X-rays, electrocardiographs and even electrokymographs, a technology that showed the image of a beating heart but was destined for the medical scrap heap.
For Evelyn, now 94 years old, it was the first of 29 biennial exams and the beginning of a three-generation involvement with Framingham. Her family’s story reads like a brief history of American health care during the second half of the twentieth century. Aurele, a lifelong smoker, had his first heart attack in 1963, at age 55, and though he then gave up cigarettes and took up walking for exercise, his doctors didn’t know to advise him to lay off the bacon and eggs, and he died in 1976 after several more heart attacks. A son, Ronald DesRosiers, now 71, joined Framingham in 1971 and was also a smoker—startlingly, on the recommendation of a doctor who suggested he start after a bout of asthma. Eventually, though, he became more health-conscious, working with his physicians to reduce his cholesterol and control his hypertension. His sister, Anne Marie Bouchard, and her husband, Armand, who return regularly from Maine for Framingham exams, have embraced the study’s findings. They’re longtime walkers, sailors and cross-country skiers, and they make sure to eat healthy foods. Their daughter, Sharon Wade, 41, who jumped at the chance to become a third-generation Framingham participant, also watches her diet, exercises several times a week and appreciates being on the front lines of preventive medicine. “If there’s a new test to assess risk of stroke or heart attack, we’ll get it at Framingham,” Wade says.
That, of course, is the Framingham bargain: Those who participate will get the best medicine has to offer and in turn will provide data that helps expand medicine’s frontiers. The decision to recruit the children and grandchildren of the study’s original participants—adding 5,124 second-generation subjects in 1971 and 4,095 from the third generation in 2002—only extended that covenant of mutual interest in the hope of breaking still more ground. These days, that inevitably involves going beyond risk factors to seek to understand the genetic root causes of disease.
That would have been an unimaginable concept when the study was launched. “DNA hadn’t even been discovered,” Kannel says. “All we knew back then was that certain diseases might be inherited.” Starting in the mid-1990s, Framingham researchers began collecting DNA samples from participants, and in 2003 the Human Genome Project, run by the U.S. Department of Energy and the National Institutes of Health, announced it had identified the 20,000 genes in human DNA and determined the correct order of the four chemical units found in DNA’s base pairs—abbreviated as A, T, C and G—that constitute each of the 23 chromosome pairs of a human cell. Those achievements meant that more than a half century of tracking families and disease could now be put to a new, possibly revolutionary use.
WHEREAS ALL HUMANS SHARE an identical sequence of base pairs (AT or GC) in most parts of their genome, once every 1,200 base pairs or so the sequence will differ—for example, the letters might be transposed. Those points of variation are known as single nucleotide polymorphisms, or SNPs (pronounced “snips”), and where there’s a SNP on the long strands of DNA known as chromosomes, there may be a gene implicated in a disease-causing mutation. So if people with diabetes, for example, share the same aberration of chemical base pairs at a particular SNP, researchers will look at that piece of the chromosome for one of the many genes thought tocontribute to the disease.
Even before publication of the human genome—and the subsequent International HapMap Project, completed in 2005, which identified haplotypes, chromosomal “neighborhoods” in which certain genetic variants cluster—Framingham was trying to mine its family data to identify genetic variations. Today Framingham’s geneticists can be much more precise, scanning the entire genome using gene chips that test subjects’ DNA along thousands of SNPs. “Ten years ago, we could look at only 350 genetic markers at a time,” says L. Adrienne Cupples, professor of biostatistics at Boston University School of Public Health. “Now we have a 1-million-SNP chip.”
Last September the Framingham study released an analysis of its first genomewide association study, which tested DNA from 1,345 Framingham participants against 100,000 SNPs. Researchers looked for associations between the SNPs and what they knew about the people being tested. They considered 987 individual characteristics known as phenotypes—lipid levels, blood pressure, bone mass, presence or absence of cancer or diabetes, and so on—to determine which genetic variations may cause or contribute to those traits. So if, for example, hypertensive Framingham participants have a SNP that reads ATCG, but the SNP of those with normal blood pressure reads attg, that’s evidence the SNP is associated with hypertension. The next step is to hone in on that area to find SNPs that the genome scan didn’t detect because there are often multiple mutations clustered together. Once they know the full scope of the variation, researchers can try to find the real functional mutation—the gene.
THE 100,000-SNP SCAN WAS CONDUCTED in early 2006; Framingham followed up in 2007 with a genome-wide association scan covering 550,000 SNPs—80% to 90% of the locales on the genome at which geneticists think they’re likely to discover disease-causing genes. Called the SHARe (SNP Health Association Resource) Project, the scan tested the DNA of more than 9,000 Framingham participants from three generations. (The analysis won’t be complete for another six to 12 months.)
As the acronym implies, the results from SHARe will be available to qualified researchers around the world via an online database developed by the National Library of Medicine’s National Center for Biotechnology Information. “Many more discoveries will come from this research if we collaborate with researchers conducting other genome studies, which is absolutely critical to moving the field forward,” O’Donnell says.
Because Framingham may well have collected more data on its subjects’ physical traits than any other study in the world, it is poised to find genes causing dozens of diseases and could also help solve the mystery of how multiple genes interact—enabling scientists to understand, say, the effect that genes regulating insulin have on those controlling cholesterol. “How fast we can come up with a therapy to alter the expression of genes for a particular disease will depend on how many SNPs affect the risk of heart disease, for instance,” Cupples says. Researchers already know there are many genes involved, but if some of those genes lie on the same pathway, blocking that path with a drug might lower a person’s risk for heart disease.
So far, Framingham researchers speak guardedly about the results of their first scan, saying only that it confirmed the effects of some genes that were already suspected of contributing to various conditions and provided “interesting leads” to generate hypotheses about where to search for others. “Did we find something terribly exciting? No,” says Larry Atwood, professor of neurology at Boston University and a geneticist for FHS. “But this is basic science and we have to creep along incrementally.”
Nor is it possible to draw conclusions from one scan. “It’s the nature of statistics that some results that seem biologically significant will turn out to be random fluctuations,” Atwood says. And the greater the number of SNPs tested, the more likely that is to occur. But that’s another area in which Framingham’s family-based study provides an advantage. When researchers know that several generations show the same genetic variation, they can be much more confident in their findings.
Still, Atwood cautions that it may take years before scientists fully understand the genetic architecture of some diseases. Whereas genome scans for diabetes, for example, have seemed relatively definitive, revealing as many as eight genes that appear to have a significant effect on blood glucose, hypertension remains a genetic mystery. “Many systems in the body appear to have an effect on blood pressure, which means there may be very many genes involved,” Atwood says.
There’s also the matter of determining the relative effect of implicated genes. “If the eight we’ve identified for diabetes have a large effect on the disease, then we’re nearly done with the search,” Atwood says. “But if there are 40 more, each affecting variation in blood glucose in a small way, we’re still looking at an extremely difficult problem.”
IN THE DECADES SINCE FRAMINGHAM first introduced disease risk factors to medical parlance and practice, death rates for coronary heart disease and stroke have dropped 60% and 66%, respectively. Studies suggest that about half that reduction has been the result of preventive medicine and a population that has consciously altered its behavior; better medical procedures and treatments are credited with the rest of the improvement. Now there’s the matter of how discoveries about genetics may intersect with the risk factors on which Framingham has built its legacy. “It’s a good question and one that Framingham would be perfect to research,” O’Donnell says. “I suspect we’ll find that a genetic predisposition to certain diseases, like other risk factors, can still be countered by lifestyle changes; but it may mean those changes have to be particularly aggressive.”
It’s possible that the study will recruit a fourth generation of Framingham participants, perhaps even before they reach adulthood, to find the seeds of disease long before they take root. “It’s not reasonable to assume that the Framingham Heart Study will go on forever,” Levy says. “But as long as it continues to ask the right questions, identifies methods to answer them and generates information to improve public health, it will last.” Adds Wolf: “There’s a lot of gold in Framingham left to mine.”
Dossier
A Change of Heart: Unraveling the Mysteries of Cardiovascular Disease, by Daniel Levy and Susan Brink (Vintage Books, 2005). A behind-the-scenes history of the people, science, politics and culture that created one of the longest-running epidemiology studies.
“You Changed America’s Heart: A 50th Anniversary Tribute to the Participants in the Framingham Heart Study.” A report that both enumerates the medical accomplishments of the study and offers the human side with anecdotes from participants.
“The Hidden Epidemic: Heart Disease in America,” PBS, February 2007. In a far-reaching and candid interview with Larry King, William Kannel, a former FHS director, discusses the early days of the Framingham study, how its findings influenced medical practices over the years and why heart disease remains a major problem in America.
Stay on the frontiers of medicine
Related Stories
- In Framingham, Deep Cuts
Sharply pared budgets could kill the Framingham Heart Study—after 50 years of astonishing research breakthroughs.
- Going the Distance
Longitudinal studies have provided both puzzles and insights about human health and well being.